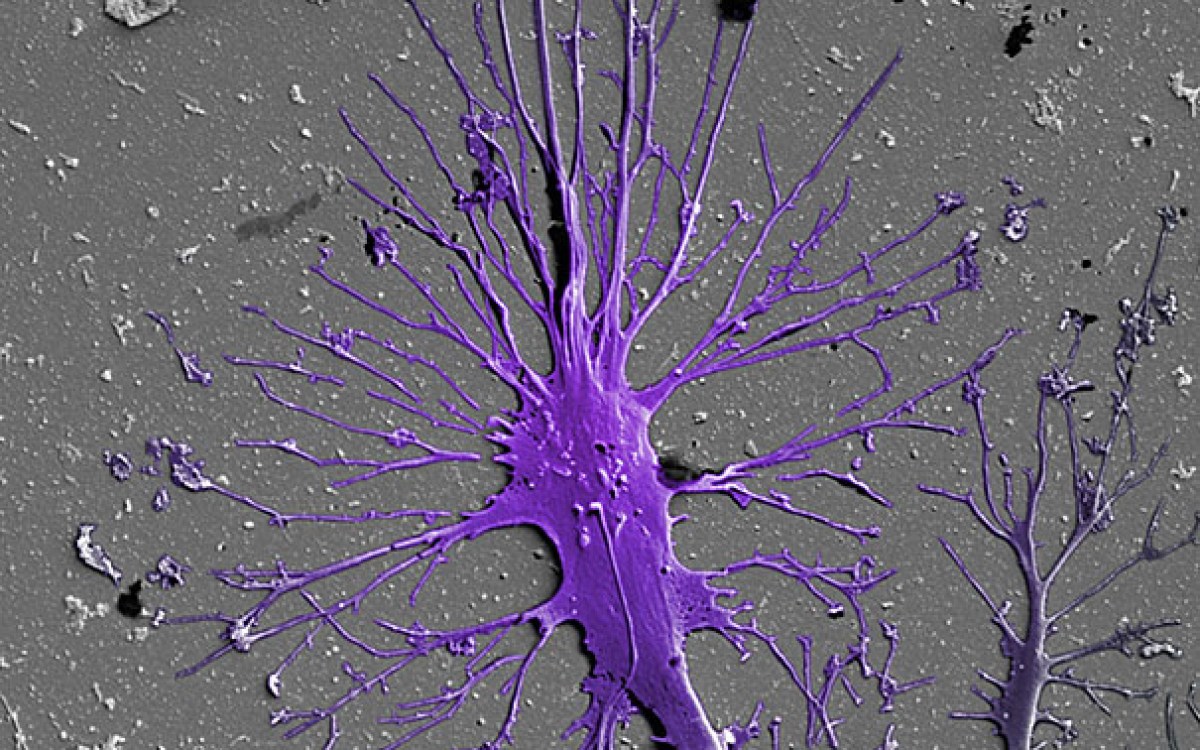
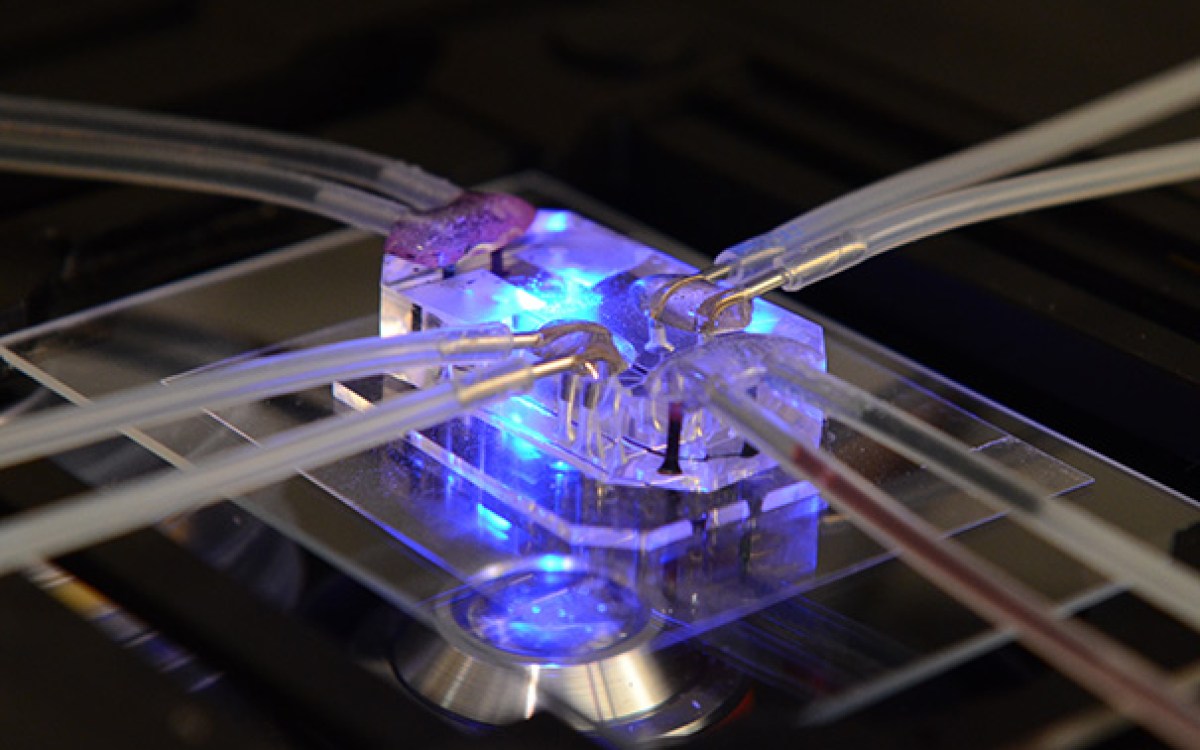
Engineered mini-kidneys come of age
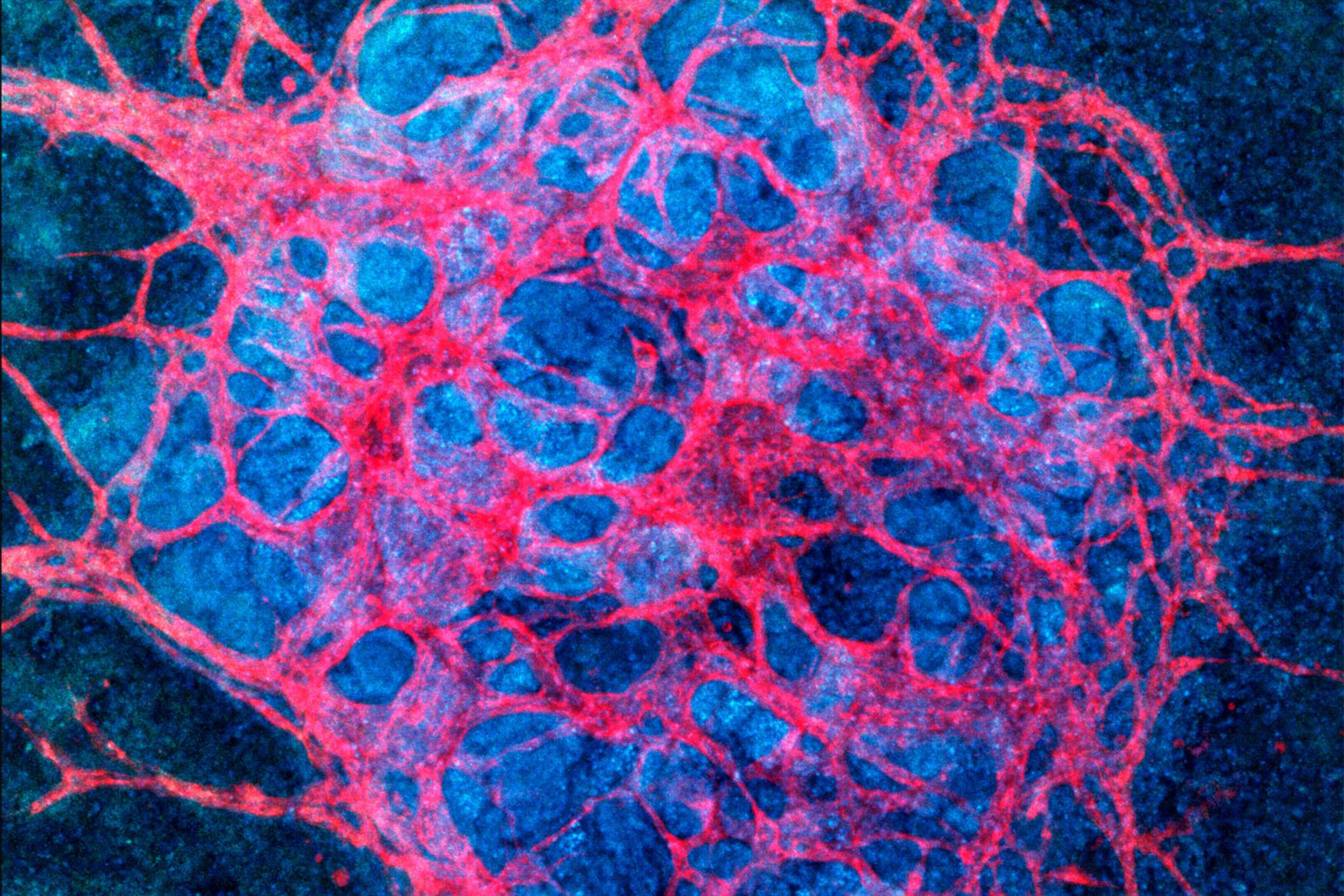
The ability to culture kidney organoids, thus creating more mature vascular networks will open up “new avenues for accurately testing drug toxicity in vitro … and modeling kidney diseases …,” says Jennifer Lewis, co-leader of the research team.
Credit: Wyss Institute at Harvard University
Growing organoids under flow increases their potential for drug testing, regenerative medicine
With organs-in-a-dish a growing success story, research with organoids has increasingly proved its worth. Already, scientists can create organoids that have many of the cell types and complex architectures of human organs such as the kidney, liver, guts, and even the brain.
Most organoids grown in vitro , however, have lacked the vasculature to provide the cells with oxygen and nutrients, remove metabolic waste, and facilitate communication between cell types — functions that drive their maturation into working tissue-building blocks.
When it comes to kidney organoids, that shortcoming has kept researchers from reproducing key functions, such as blood filtration, reabsorption, and urine production. A vascularized organoid could better model kidney diseases, enhance renal drug toxicity testing, and ultimately lead to building blocks for replacement therapies.
To answer that need, a team of researchers from the Wyss Institute for Biologically Inspired Engineering, the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), Brigham and Women’s Hospital, and the Harvard Stem Cell Institute has developed a powerful new approach as part of the Wyss’ new 3D Organ Engineering initiative. By exposing stem cell-derived organoids to fluidic shear stress, they have significantly expanded their vascular networks and improved the maturation of kidney compartments. The work is published in Nature Methods.
The improvements are a next step on a path begun in 2015, when Ryuji Morizane and Joseph Bonventre developed a way to make kidney organoids from human pluripotent stem cells. But while their organoids had well-organized nephrons, which filter the blood, and primitive blood vessels, “they still lacked pervasive vascular compartments with perfusable lumens,” said Morizane, an assistant professor at Brigham and Women’s Hospital and Harvard Medical School (HMS), a member of the Harvard Stem Cell Institute, and, with co-author Jennifer Lewis, co-leader of the research team.
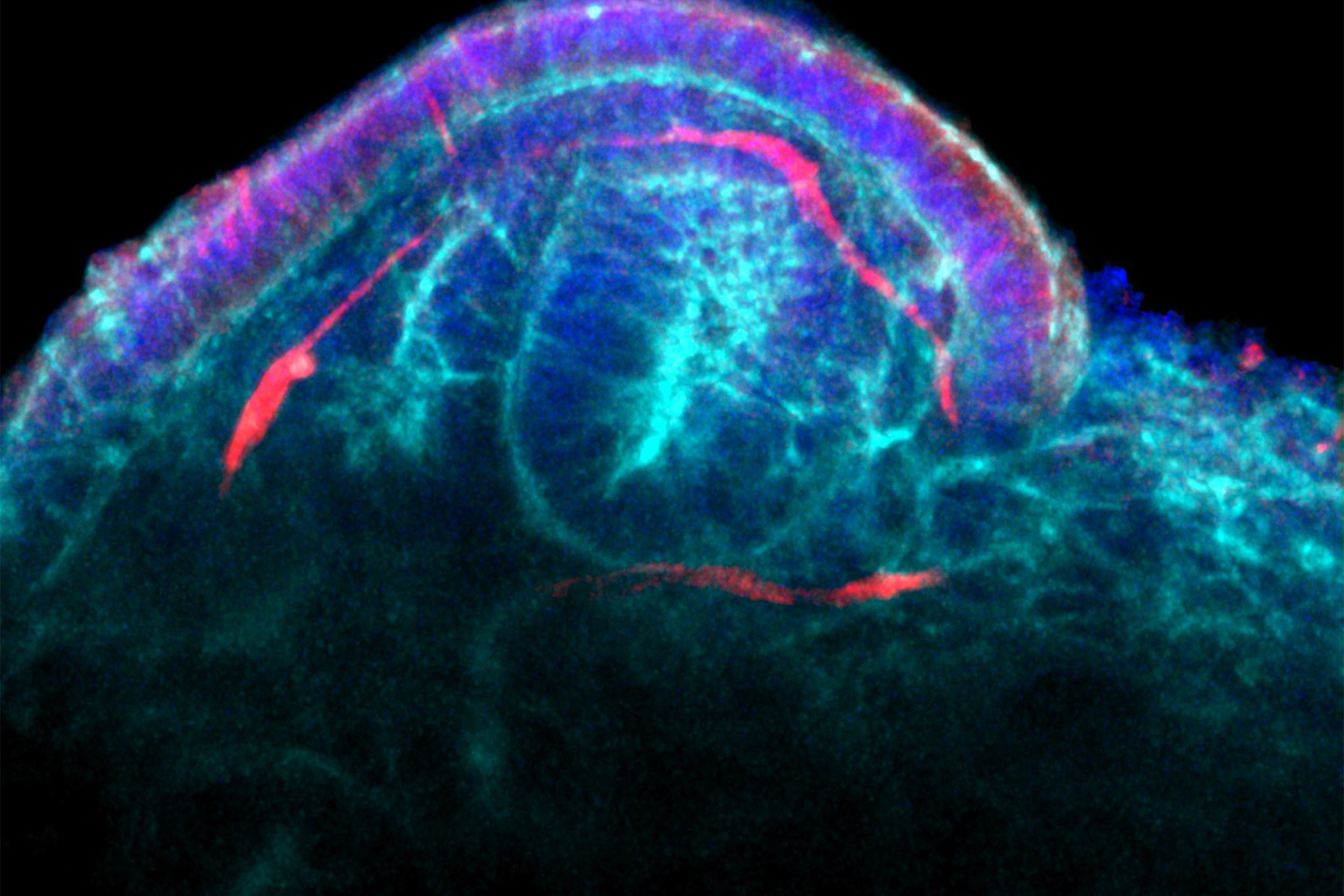
To develop these, the team used the Lewis lab’s strategies to create vascularized human tissues, including 3-D kidney-on-chip models, using perfusable and durable 3-D bioprinting. They hypothesized that fluid flow could help the chip models form blood vessels from precursor endothelial cells found in growing kidney organoids — and successfully, for the first time, demonstrated that by exposing the organoids to fluid flow, their vascularization and maturation can be enhanced in vitro, rather than in an animal host, said Morizane.
“We determined the right combination of underlying extracellular matrix, media additives, and fluidic shear stress under which human stem-cell derived organoids would flourish when grown in our 3-D-printed millifluidic chips,” said Kimberly Homan, a research associate in Lewis’ group and, with Navin Gupta, first author on the study.
“The vascular networks form close to the epithelial structures that build the glomerular and tubular compartments, and in turn promote epithelial maturation. This integrated process works really like a two-way street,” said Gupta, a clinical research fellow in Morizane’s lab.
“This important advance opens up new avenues for accurately testing drug toxicity in vitro … and modeling kidney diseases, like polycystic kidney disease, that affect specific structures and cell types using patient-derived stem cells as the starting point,” said Lewis, who is a core faculty member of the Wyss Institute and co-leader of its 3D Organ Engineering Initiative. Lewis is also the Hansjörg Wyss Professor of Biologically Inspired Engineering at SEAS and a member of the Harvard Stem Cell Institute.
“Our method may pave the way to also vascularize other types of organoids, such as the liver organoids,” she said.
“This study is a great example of the importance of mechanobiology and the potential power of the Wyss Institute’s 3D Organ Engineering Initiative. It provides an important cornerstone for many efforts that aim to create functional human tissues de novo for research, pharmaceutical, and tissue regenerative applications,” said Wyss Institute Founding Director Donald Ingber, who is also the Judah Folkman Professor of Vascular Biology at HMS and the Vascular Biology Program at Boston Children’s Hospital, as well as professor of bioengineering at SEAS.
Other authors on the study are Bonventre, the Samel A. Levine Professor of Medicine at HMS, chief of renal medicine at the Brigham, and a member of the Harvard Stem Cell Institute; past and present members of Lewis’ team, including Katharina Kroll, Mark Skylar-Scott, David Kolesky, Donald Mau, and Thomas Ferrante; Tomoya Miyoshi on Morizane’s team; and M. Todd Valerius, principle investigator at the Brigham.
The study was funded by the National Institutes of Health, Harvard’s Wyss Institute for Biologically Inspired Engineering, and the Harvard Stem Cell Institute.