How cells repair damage to DNA
Scientists’ findings shed light on repair of mutations that can elevate cancer risk
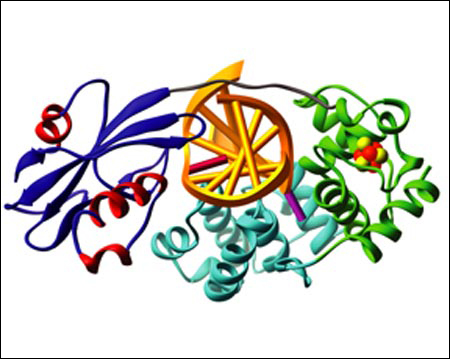
Harvard University scientists have found a mechanism by which cells repair a key type of oxidative DNA damage that, left unchecked, can lead to a greatly elevated risk of colon cancer. The findings appear in the Feb. 12 issue of the journal Nature.
While the scientists caution that the work is unlikely to provide immediate clinical applications, their research represents an important step in understanding how cells avert genetic mutations by resisting the toxic effects of free radicals, agents to which humans are constantly exposed and which have been strongly implicated in cancer and aging.
“We have elucidated the mechanism by which a cellular protein, MutY, repairs an especially important type of DNA lesion resulting from accidental replication of a damaged DNA base,” says author Gregory L. Verdine, Erving Professor of Chemistry and Harvard College Professor. “This process is especially thorny because MutY is actually plucking out an otherwise normal DNA base, adenine, which is abnormal only in that it is paired with a damaged base.”
Humans and other animals need oxygen to survive, but in recent years researchers have become increasingly aware of the damage that can be wrought by uncontrolled oxidation. In consuming oxygen the body produces reactive intermediaries that are ferocious oxidizers. These so-called “free radicals” bind to anything and everything they encounter, including DNA and other key cellular components.
Free radicals attach preferentially to the DNA base guanine, and this oxygen-bound guanine can derail the cell’s DNA-replicating machinery. Specifically, an oxidized guanine’s partner base, normally cytosine, will be miscopied by the cell’s replication machinery as adenine. Over time such mutations can accumulate, a process that scientists believe leads to cancer and other attendants of the aging process.
Verdine and graduate students Chris Fromme and Anirban Banerjee of Harvard’s departments of Molecular and Cellular Biology and Chemistry and Chemical Biology, respectively, determined how cells can fix such errors. When MutY comes across this mismatched base pair of an oxidized guanine and an adenine, it cleaves the adenine so the improper base can be replaced by cytosine. Other proteins that are part of the same DNA-repairing family then kick in to remove the oxidized guanine that caused the problem in the first place, returning the strand of DNA to its original, pre-oxidation condition.
“It has recently been shown that people who inherit certain rare forms of MutY are highly susceptible to colon cancer,” Verdine says. “Our work explains why these rare forms are ineffective at repairing DNA, thereby allowing mutations to accumulate and cancer to develop.”
Banerjee developed a novel technique for securing MutY to DNA, using disulfide cross-linking in visualizing the crystal structures of MutY-DNA complexes. Fromme then determined the exact structure of MutY as it binds to DNA. This technique is similar to one used previously by the same laboratory to stabilize HIV reverse transcriptase on DNA, which led to the determination of a structure that has been widely used to design new AIDS drugs.
Fromme, Banerjee, and Verdine were joined in the research by Susan J. Huang, an undergraduate in Harvard’s Department of Molecular and Cellular Biology. Their work is supported by the National Institutes of Health and the U.S. Department of Energy.