A win for science, and patients, against brain injury ‘nihilism’

Illustrations by Liz Zonarich/Harvard Staff
Hope for progress even after a 450-foot fall, trial shows, defying pessimism that hurts research and families
New research may upend the widely held view of traumatic brain injury as a permanently debilitating condition. The findings indicate that electrical stimulation can reawaken quiescent brain circuitry, leading to functional improvements that have the potential to restore work and social activities to patients’ lives.
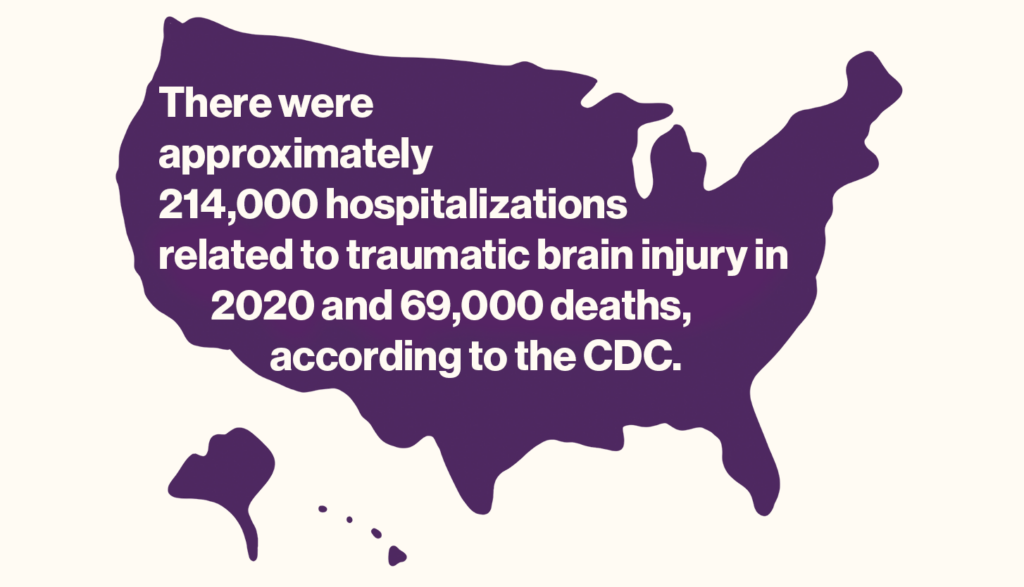
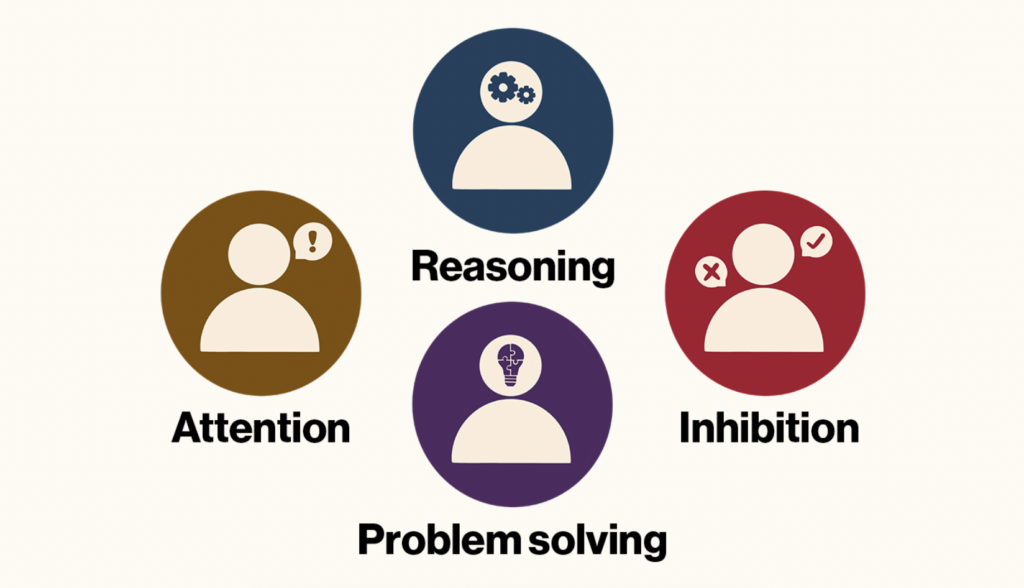
The early stage trial, by investigators at Harvard-affiliated Spaulding Rehabilitation Hospital and colleagues at institutions around the country, including Weill Cornell Medicine, Stanford University, the Cleveland Clinic, and the University of Utah, was completed by five of the six enrolled patients. It yielded improvements of between 15 percent and 52 percent on a standard test of executive function, which involves attention, inhibition, reasoning, problem-solving, and other key aspects of mental processing.
Examples of executive function
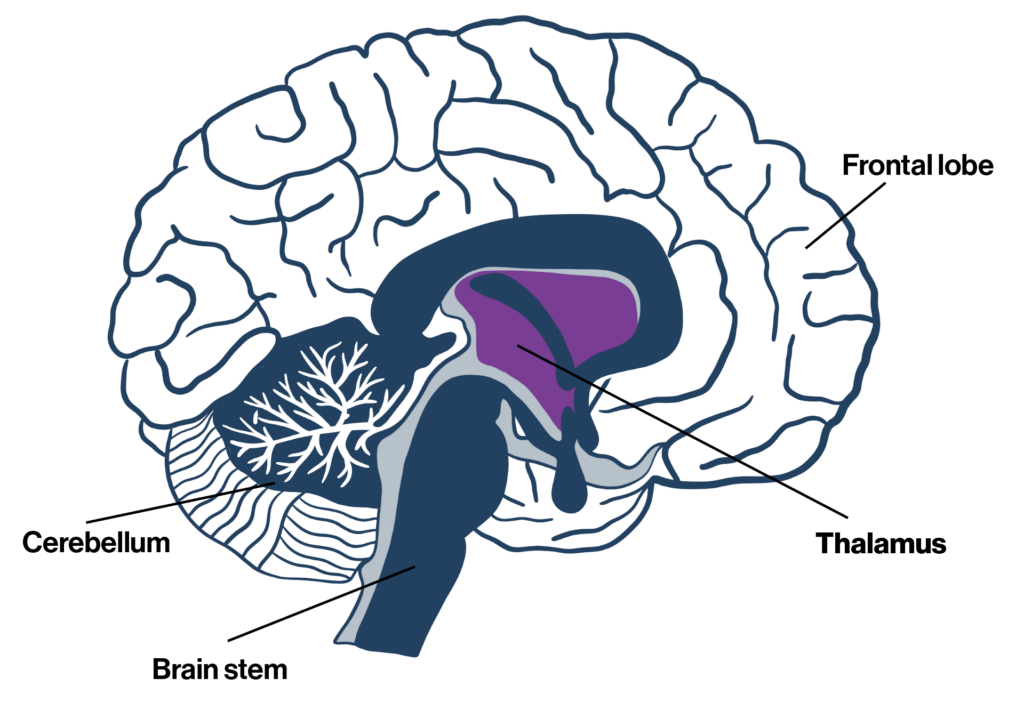
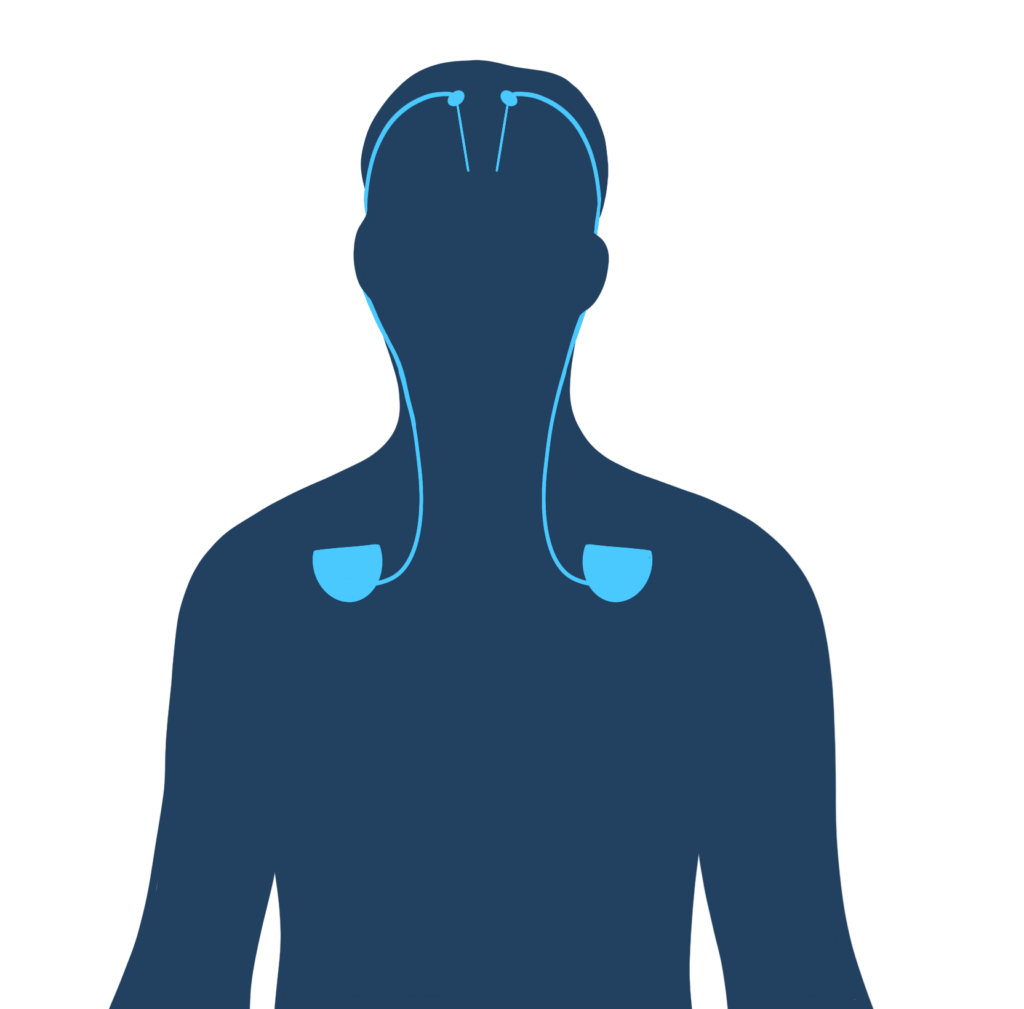
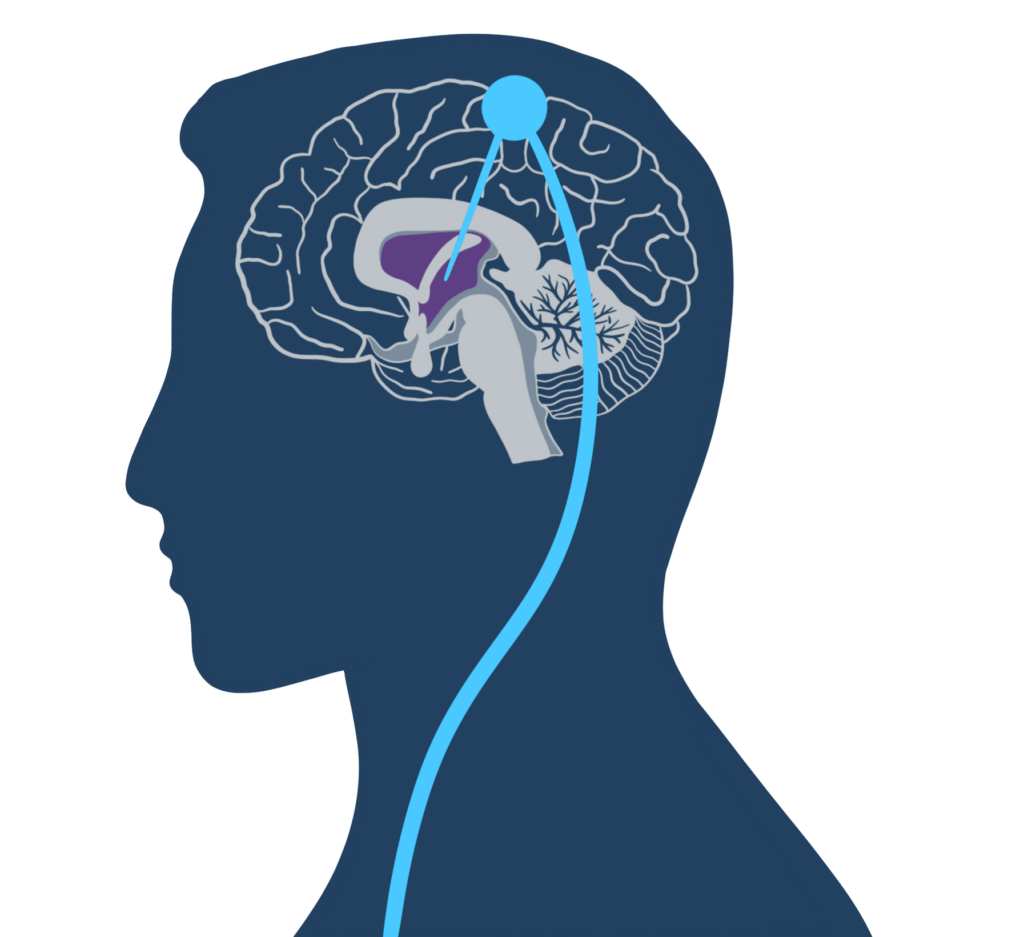
The study, published in December in Nature Medicine, relied on “deep brain stimulation,” in which a battery-powered device is implanted under a patient’s skin. Electrodes extend from the device to a part of the thalamus, which routes signals from one section of the brain to another.
“There’s a lot of ways to shut parts of the system down,” said Joseph Giacino, Spaulding’s director of rehabilitation neuropsychology and a professor of physical medicine and rehabilitation at Harvard Medical School, who helped design the study. “One is by direct damage to specific structures. Then you have the more common situation where I can damage the pathways that connect those structures and I get the same effect.
“If the thalamus — this key relay station and signaling system — is damaged, it can’t activate or upregulate those circuits that are relatively spared and could work and perform their role if they had the right input.”
The volunteers had suffered moderate to severe brain injury from either a motor vehicle accident or a fall — one from 450 feet and another from roughly 60 feet. The accidents occurred between three and 18 years earlier, long enough that the victims were considered past the immediate post-injury phase when most healing takes place. Each had recovered enough to perform the activities of daily living — personal hygiene, dressing, and feeding themselves — but had not regained pre-injury levels of work, study, and social activities.
A common view of brain injury, held by both laypeople and the medical community, is that the affected cells cannot regrow, leading to permanent disability. In this case, investigators tested an alternate theory: The idea that some — perhaps many — injuries disrupt signaling between parts of the brain, and it is the loss of communication, rather than cell death, that causes much of the decline in function. If that’s the case, they say, then it’s possible that stimulating brain regions important in communication can restore some function.
The five volunteers received deep-brain stimulation for 12 hours a day for three months. To gauge results, the researchers selected a standard test of executive function called the “trail making test part B” and established 10 percent improvement as a clinically relevant threshold.
By the study’s end, all five patients had exceeded the 10 percent threshold, producing an average improvement of 31.75 percent. The greatest gains, more than 40 percent, were seen in the two who had suffered falls and had the deepest initial deficits. But even those with mild impairment improved by more than 20 percent. Two participants, one injured in a car accident and a second hit by a car while riding a bicycle, regained the ability to work, albeit at reduced capacity, and socialize. The functional status of the other volunteers remained stable.
How does the device work?

One mother called her daughter’s improvement “profound” and “a miracle.” None of the participants were “cured,” however. Rather, the implant plays a long-term role analogous to that of a cardiac pacemaker. Even though the trial’s experimental phase concluded years ago, all five participants still have their devices. Some have had new batteries installed.
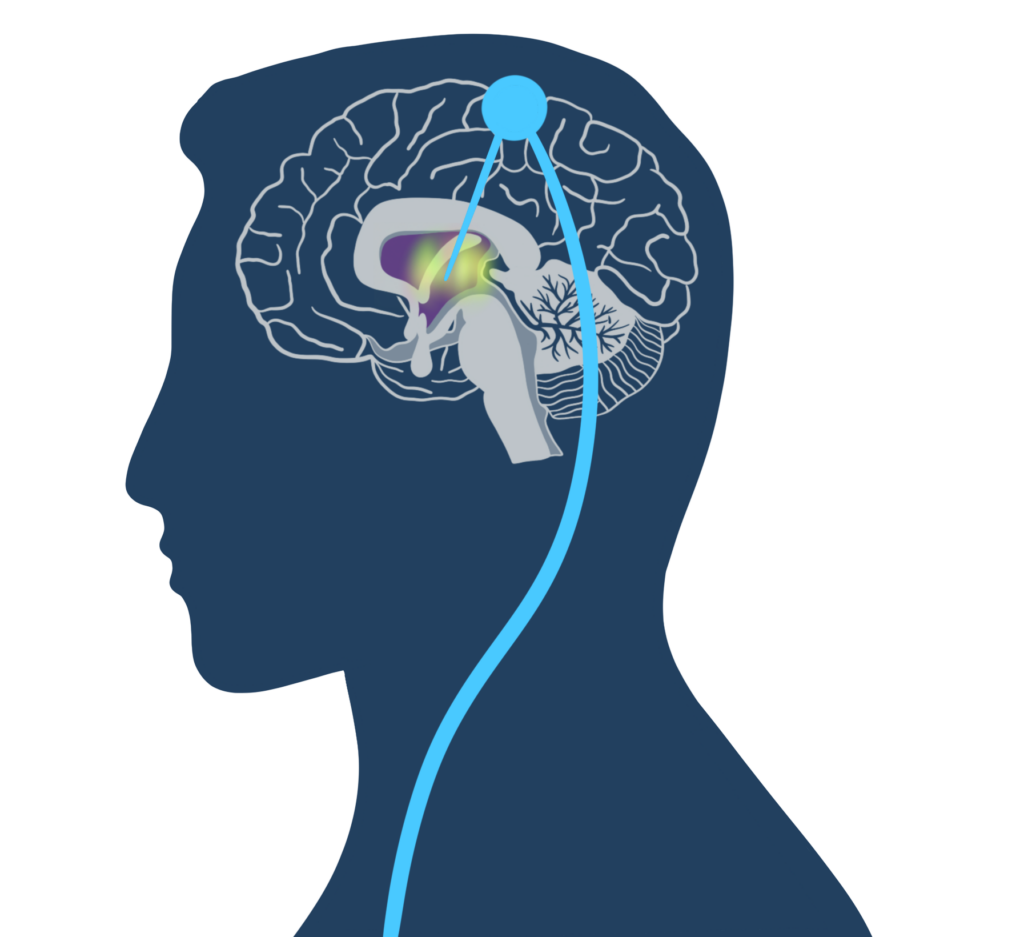
The researchers don’t understand precisely how deep-brain stimulation improves functioning, but they are skeptical that the artificial signals replace neural messages. The team’s favored explanation, Giacino said, is that the stimulation serves to activate the thalamus, which in turn upregulates viable downstream parts of the brain, restoring at least some signaling capacity.
“I think what it’s doing is putting the brain into a state of readiness, so when the demands are there to engage a particular network or subsystem, it can do that,” he said.
Patients in the study saw improvements to capabilities that had been considered set in stone for as long as 18 years. This doesn’t mean that deep-brain stimulation is likely to be a universal answer, Giacino noted. And though the work suggests that not all loss of function is due to cell death in specific parts of the brain, some of it undoubtedly is. But overall, the results suggest that it’s time to rethink the idea that brain injury is permanent, he said. A Phase 2 study, set to involve about 50 people, is now in the planning stages.
“The degree of nihilistic belief that exists, both among the lay public and clinicians who see persons with severe brain injury, is remarkable and hugely problematic,” Giacino said. “This belief that if you have a severe brain injury you’re fated to do poorly — you’ll get some period of time in which your brain will naturally try to heal itself and then you’re at your final outcome — has been a problem for clinical practice, for research, for funding, and for people who have severe brain injury and their families.”
The progress of patients in the trial, he added, “helps chip away at the nihilism that really makes our work so much more difficult.”