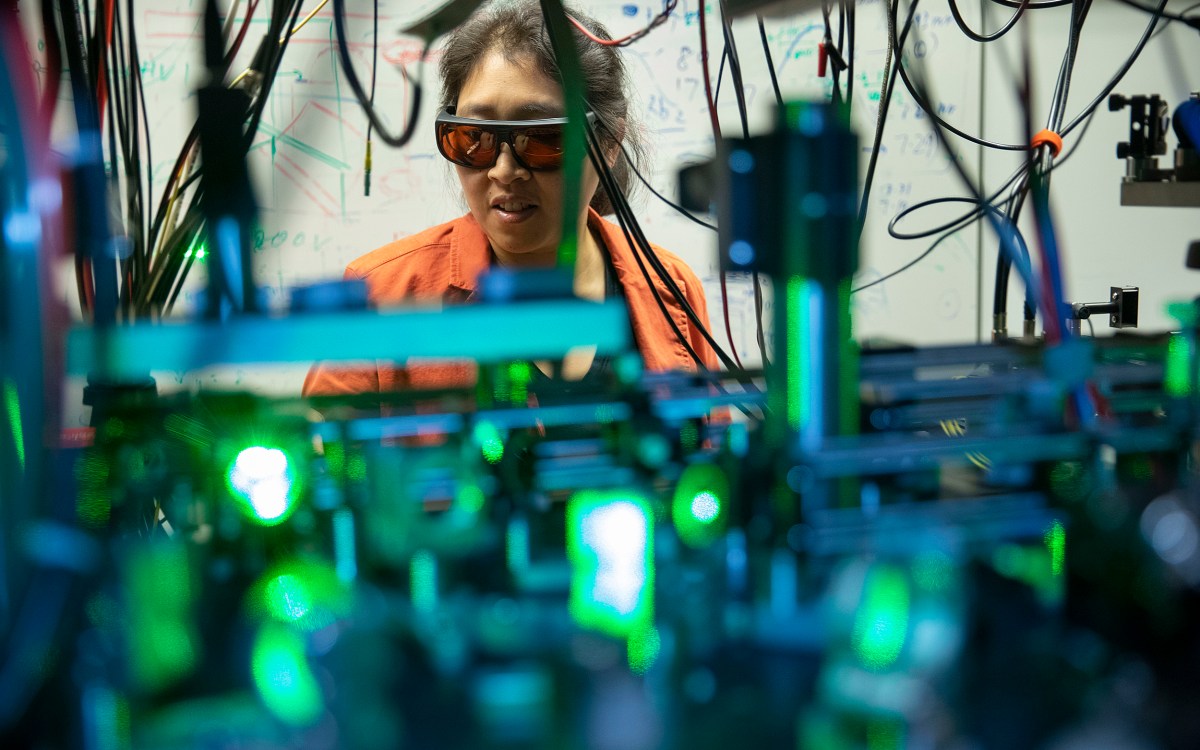
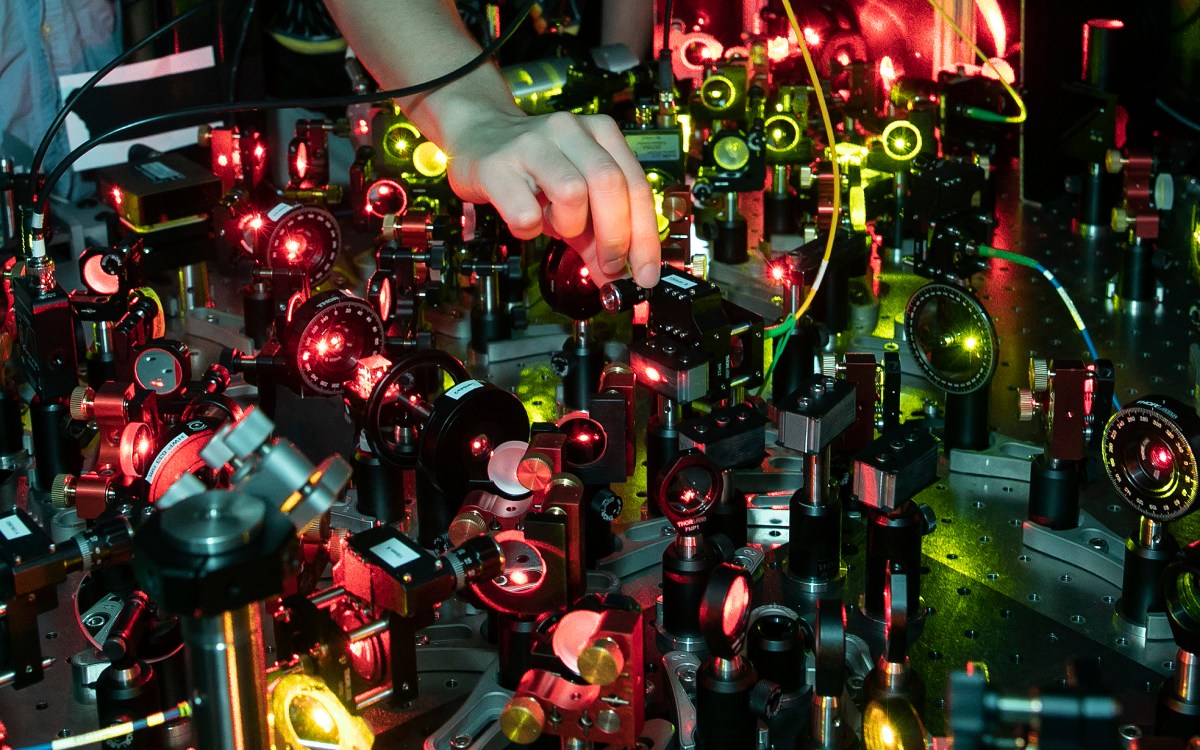
Professor Kang-Kuen Ni and her team have not only watched chemical transformations occur, but now they’ve found the missing molecules in the process.
Kris Snibbe/Harvard file photo
Mystery of the missing molecules
Researchers crack case by trapping ultracold molecules in mid-chemical reaction
In a famous parable, three blind men encounter an elephant for the first time. Each touches a part —the trunk, ear, side — and concludes the creature is a thick snake, a fan, or a wall. This elephant, said Kang-Kuen Ni, is like the quantum world. But scientists understand that they can only explore one tiny bit of this vast, unknown creature at a time. Now, Ni has revealed a few more to explore.
It started last December, when she and her team constructed a new apparatus capable of achieving the lowest-temperature chemical reactions of any currently available technology, and then broke and formed the coldest bonds in the history of molecular coupling. An unforeseen benefit was that the ultracold temperatures slowed the reaction so much that researchers caught the first real-time glimpse of what happens during a chemical transformation. Though reactions were considered too fast to measure, Ni managed to determine the lifetime of that one — and solve the mystery of the missing molecules in the process.
With ultracold chemistry, Ni, the Morris Kahn Associate Professor of Chemistry and Chemical Biology and of Physics, and her team cooled two potassium-rubidium molecules to just above absolute zero and found the “intermediate,” the space where reactants transform into products, lived for about 360 nanoseconds (almost a million times longer than they live in higher-temperature reactions). “It’s not the reactant. It’s not the product. It’s something in between,” Ni said. Watching that transformation, like touching the side of an elephant, can tell her researchers something new about how molecules, the foundation of everything, work.
But they didn’t just watch.
“This thing lives so long that now we can actually mess around with it … with light,” said Yu Liu, a grad student in the Graduate School of Arts and Sciences and first author on the study published in Nature Physics. “Typical complexes, like those in a room-temperature reaction, you wouldn’t be able to do much with because they dissociate into products so quickly.”
More like this
Like “Star Trek” tractor beams, lasers can trap and manipulate molecules. In ultracold physics, this is the go-to method for capturing and controlling atoms, observing them in their quantum ground state, or forcing them to react. But when scientists moved from manipulating atoms to messing with molecules, something strange happened: Molecules started to disappear from view.
“They prepared these molecules, hoping to realize many of the applications that they promise — building quantum computers, for example — but instead what they see is loss,” Liu said.
Alkali atoms, like the potassium and rubidium Ni and her team study, are easy to cool down in the ultracold realm. In 1997, scientists won a Nobel Prize in physics for cooling and trapping alkali atoms in laser light. But molecules are wonkier than atoms: They aren’t just a spherical thing sitting there, said Liu. They can rotate and vibrate. When trapped together in the laser light, the gas molecules bumped against each other as expected, but some simply disappeared.
Scientists speculated that the molecular loss resulted from reactions — two molecules bumped together and, instead of heading off in different directions, they transformed into something new. But how?
“What we found in this paper answers that question,” Liu said. Turns out it’s the light’s fault.
When Liu and Ni used lasers to manipulate that intermediate complex — the middle of their chemical reaction — they discovered the light forced the molecules off their typical reaction path and into a new one. A pair of molecules, stuck together as an intermediate complex, can get “photo-excited” instead of following their traditional path, Liu said. Alkali molecules are particularly susceptible because of how long they live in their intermediate complex.
“Basically, if you want to eliminate loss, you’ve got to turn off the light,” Liu said. “You’ve got to find another way to trap these things.” Magnets, for example, or electric fields can trap molecules, too. “But these are all technically demanding,” said Liu. Light is just simpler.
Next, Ni wants to see where these complexes go when they disappear. Certain wavelengths of light (like the infrared the team used to excite their potassium-rubidium molecules) can create different reaction paths — but no one knows which wavelengths send molecules into which new formations.
They also plan to explore what the complex looks like at various stages of transformation. “To probe its structure, we can vary the frequency of the light and see how the degree of excitation varies,” Liu said. “From there, we can figure out where the energy levels of this thing are, which informs on its quantum mechanical construct.”
“We hope this will serve as a model system,” Ni said, an example for how researchers can explore other low-temperature reactions that don’t involve potassium and rubidium.
“This reaction is, like many other chemical reactions, sort of a universe in its own,” said Liu. With each new observation, the team reveals a tiny part of the giant quantum elephant. Since there are an infinite number of chemical reactions in the known universe, there are still many, many pieces to explore.
Funding support for the project came from the Department of Energy, David and Lucile Packard Foundation, the Dutch Research Council (NWO), and the National Science Foundation, Department of Defense, and the Alexander von Humboldt Found