Joshua Levin (from left), Xian Adiconis, Paola Arlotta, Aviv Regev, Silvia Velasco, Amanda Kedaigle, and Marina Rocha are scientists researching a major advance in the development of human brain “organoids.”
Rose Lincoln/Harvard Staff Photographer
Forward thinking
Reproducible, miniature 3D models of human brain tissue open new frontiers in neuroscience
Researchers have made a major advance in the development of human brain “organoids” — miniature, 3D tissue cultures that model brains in a dish. The new method, published in Nature, consistently grows the same types of cells, in the same order, as the developing human cerebral cortex. The advance could change the way researchers study neuropsychiatric diseases and test the effectiveness of drugs.
The genetics behind human neurological disease are complex, with large spans of the genome contributing to disease onset and progression. Studying neurological diseases in other animals provides limited opportunities for relevant discovery, as human brains are quite distinctive.
Organoids — simplified, multicell replicas with some of the features of the organ they model, organoids allow scientists to see how cell types within a structure interact with one another — offer great promise for studying human disease directly. But so far, they have failed in one very important way: They’re inconsistent.
“We might all use our brains differently, but each of us has the same collection of cell types and basic connections,” said senior author Paola Arlotta, the Golub Family Professor of Stem Cell and Regenerative Biology at Harvard and a member of the Broad’s Stanley Center for Psychiatric Research. “That consistency is crucial and, with very few exceptions, it is reproduced every time the human brain forms in the womb. There are only the smallest differences between us in terms of the cell types and structures in our brains.”
So far, that has not been the case with organoids. While they do generate human brain cells, each one is unique. That means they cannot be used easily to compare differences between diseased and control brain tissues reliably.
“Organoids have dramatically advanced our ability to study the human developing brain,” said Arlotta. “But until now, each one has been its own snowflake, making its own special mix of cell types in a way that could not have been predicted at the outset. We solved that problem.”
Building on seminal work led by the late stem cell biologist Yoshiki Sasai, the team created organoids that are virtually indistinguishable from one another — even when grown for longer than six months in the laboratory.
Furthermore, under specific culture conditions the organoids were healthy and able to develop long enough to produce a broad spectrum of the cell types normally found in the human cerebral cortex.
These advances mean that brain organoids can now be used as viable experimental systems to study diseases in patient tissues directly, and to compare the effects of various drugs. “Human brain organoids create the opportunity to understand human brain development and provide a critical model, mediating between dispersed cell cultures and animals to study many devastating neurodevelopmental disorders,” said Steven Hyman, core institute member and director of the Stanley Center. “The utility of this model has been limited by its variability until now. The Arlotta lab has taken a giant step toward making brain organoids into much-needed models in which to study human brain disease.”

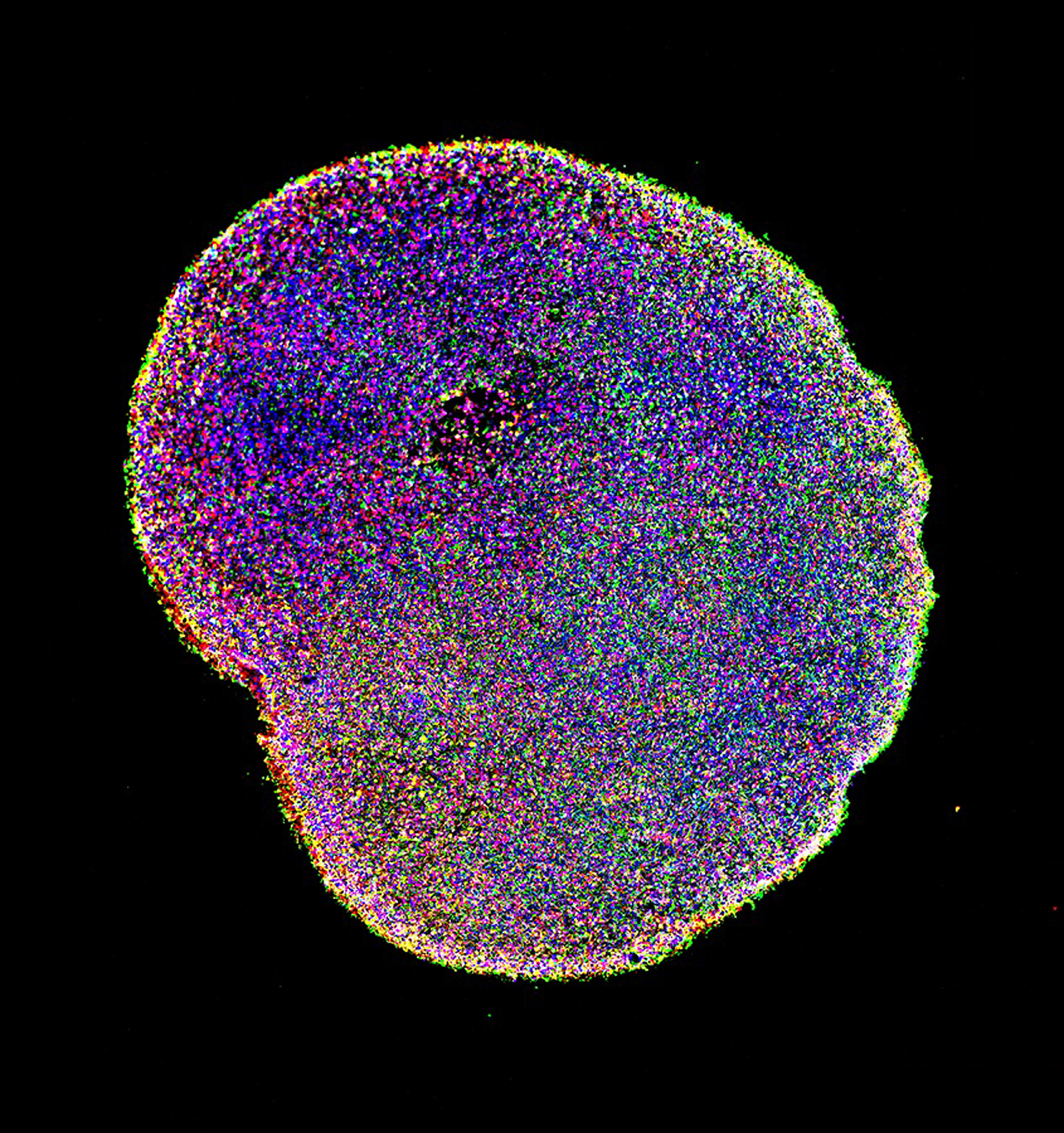
After one month of growing in the lab, a brain organoid contains clusters of cells that will develop into neurons. After six months, a brain organoid contains multiple types of neurons shown in different colors, representing the complexity of the human brain.
Paola Arlotta laboratory, Harvard University
The same cells, the same way
The researchers focused on organoids of the cerebral cortex — the part of the brain responsible for cognition, language, and sensation. The cerebral cortex plays a key role in neuropsychiatric diseases such as autism spectrum disorder and schizophrenia.
“We made organoids from multiple stem cell lines, from both male and female origins — so their genetic backgrounds were different,” said lead author Silvia Velasco, a research scientist at Harvard and the Broad Institute.
Human brain tissues grow very slowly. In this study, after six months the organoids had grown to three millimeters across. In the largest single-cell RNA sequencing experiment in brain organoids to date, the researchers grouped cells based on which genes were expressed at various stages. Using computational models for big data analysis, they compared each group to the cell types that develop in the embryonic cerebral cortex.
“Despite the different genetic backgrounds, we saw that the same cell types were made in the same way, in the correct order and, most importantly, in each organoid,” said Velasco. “We were really excited that this model gave us such consistency.”
A new way to investigate disease
Using the optimized method from this study, researchers could make organoids from stem cells derived from patients, or engineer cells containing mutations that are associated with specific diseases.
Arlotta’s lab is currently exploring autism, using CRISPR/Cas9 gene-editing techniques to develop brain organoids specific to the disorder.
“It is now possible to compare ‘control’ organoids with ones we create with mutations we know to be associated with the disease. This will give us a lot more certainty about which differences are meaningful, which cells are affected, and which molecular pathways go awry,” said Arlotta. “Having reproducible organoids will help us move much more swiftly toward concrete interventions, because they will direct us to the specific genetic features that give rise to the disease. In the future, I envisage we will be able to ask far more precise questions about what goes wrong in the context of psychiatric illness.”
“In a short time, we have gained a remarkable amount of knowledge about the many different cell types in the human brain,” said co-author Aviv Regev, who is a core institute member and chair of the Faculty at the Broad Institute, as well as co-chair of the Human Cell Atlas project. “That knowledge has given us a foundation for creating models of this incredibly complex organ. Overcoming the problem of reproducibility opens the doors to studying the human brain in ways that would have been thought impossible just a few years ago.”
“Not only does this advance make it immediately possible to study brain diseases,” added Doug Melton, Xander University Professor at Harvard University and co-director of the Harvard Stem Cell Institute, “but the consistency and reproducibility is likely a first step in using organoids to begin to understand how brain functions develop — how sets of neurons ‘learn’ and ‘remember.’”
This work was supported by grants from the Stanley Center for Psychiatric Research, the Broad Institute of Harvard and MIT, the National Institutes of Health, the Klarman Cell Observatory, and the Howard Hughes Medical Institute.
Source article: Velasco S., et al. (2019). Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature (in press).