Blood vessel drugs halt cancer growth
Once called failures
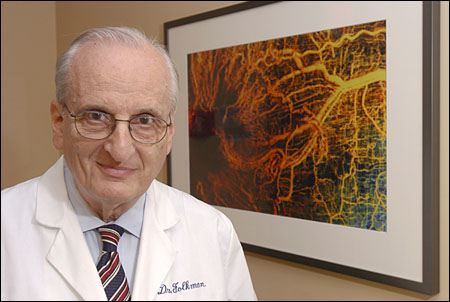
Nobody believed Judah Folkman when, in the 1960s, he claimed that the growth of cancers could be stopped, even reversed, by blocking the tiny vessels that feed them blood. Over the years, however, he has survived peer rejection of his theory, and gone on to develop drugs that did what he predicted they would do.
In 1998, endostatin, one of several anti-blood-vessel growth drugs developed in his lab, was hyped by the media as a “cure” for many different cancers. A scant seven years later, Fortune magazine derided it as a “failure.” Both statements turn out to be high exaggerations.
Talks with Dr. Judah Folkman:
- Career Real/Quicktime
- First milestone Real/Quicktime
- Isolating angiogenic factors Real/Quicktime
- Interferon Alpha Real/Quicktime
- Reversing blindness Real/Quicktime
- Professor-genesis Real/Quicktime
- Rabbi-like doctor Real/Quicktime
- House calls with Dr. KoopReal/Quicktime
A professor of pediatric surgery and cell biology at Harvard Medical School and Children’s Hospital in Boston, Folkman today is excited by what he sees in the scientific, if not the public, press. Endostatin, the drug Fortune called a failure, was used to treat 486 patients with lung cancer in China. At Dana-Farber Cancer Institute in Boston, it has given new lives to four adults who have been taking it, and it is helping children with brain and other tumors.
A related drug, called Avastin, was approved for use in the United States in February 2004. Since then, 27 other countries have OK’d it for treating colon cancer. Avastin is also being tested on patients with kidney, breast, and ovarian cancers. In addition, another blood-vessel-growth blocker, Tarceva, has been approved for treatment of lung cancer in the United States.
Researchers have also associated overgrowth of blood vessels with macular degeneration, the leading cause of blindness in elderly people. Convinced that blood-vessel-growth blockers can help these people, the Food and Drug Administration approved a drug called macugan for this purpose in December 2004. Avastin, which is made by Genentech Inc., also shows promise for treating macular degeneration.
A common antibiotic, doxycycline, was found to possess anti-blood-vessel-growth properties. So was the popular painkiller Celebrex.
Thirty to 40 other blood-vessel-growth inhibitors are now undergoing tests in patients with different types of cancers. And additional varieties of the drugs are under development to treat other diseases. Every week, Folkman sees encouraging scientific reports on the drugs he has grandfathered, but he’s not the type to gloat or scream for vindication. “It’s a large, fast-moving field,” he says modestly. “And I’m excited to see the high rate of progress.”
Ups, downs, and ups again
Folkman showed signs that he would revolutionize medicine in high school when, in the basement of his family’s Ohio home, he made a crude pump to keep a rat’s heart pumping blood. During medical training in the 1950s, Folkman and another student built an implantable pacemaker to shock weakened hearts back into a normal rhythm. But it was in 1961 when he made the discovery that now dominates his life.
Folkman and a colleague noticed that malignant mouse tumors implanted into isolated organs never grow beyond the size of a pinhead. But replant those tumors into the bodies of live animals, and they expand rapidly. From this, Folkman came up with the then-radical idea that tumors secrete proteins able to stimulate the growth of hair-thin blood vessels that bring them nutrients and carry away their wastes. He applied the name “angiogenesis,” meaning “birth of blood vessels,” to this process.
In 2005, no scientist or physician doubts the existence of angiogenesis or its major role in cancer, but in the early 1970s Folkman’s idea was heavily criticized. “Fantasy,” some experts labeled it.
By 1997, Folkman and his colleagues at Boston’s Children’s Hospital found a natural compound they called endostatin, which blocks the growth of blood vessels and shrinks tumors without the usual harsh side effects of chemotherapy.
The battle over endostatin’s efficacy as a drug, however, still rages. In 1998, the stock of the company that made the drug, EntreMed, rocketed 381 percent. But the company went broke and had to stop making endostatin.
A major problem still exists, Folkman notes, over the way doctors measure the success or failure of anti-cancer drugs. If a tumor shrinks at least 50 percent, they call it a “partial response” to the medication. Anything less becomes a “no response.” Endostatin has stabilized cancer growth, or produced slow tumor regression, over a period of three years in some patients. Although that’s less than a 50 percent response, some of the patients have gone back to work and to playing golf. Four who have been on the drug for at least 3 1/2 years are enjoying normal lives. “They were very disappointed with media reports calling endostatin a failure,” Folkman points out.
Doctors at Harvard-affiliated Children’s Hospital and Dana-Farber Cancer Institute can’t get as much of the drug as they want. Mark Kieran of Children’s Hospital is now testing endostatin against all types of solid tumors in children, including those of the brain, bone, and muscle.
The boom is on
Avastin (whose generic name is bevacizumab) enjoys good press and proves that Folkman has been on the right track for the past 33 years. “Avastin vindicates the idea that tumors can be effectively controlled by targeting the network of blood vessels that feed them,” says Joseph Paul Eder, a Harvard associate professor of medicine who treats patients at Dana-Farber Cancer Institute.
The drug has won approval from governments all over the world for its success in treating colon cancer. Once that door opened, researchers began trying it on other malignancies. Leonard Appleman is testing Avastin on kidney cancer patients at Dana-Farber. Harold Burstein, working at the same institute, has almost finished his tests of it on women with breast cancer, and says he will report his results in December. Others are treating ovarian cancer with Avastin.
No results have been formally announced, but word from people close to these trials indicates that Avastin, used by itself and in combination with other drugs, shows that the angiogenesis-blocker boom is on. One scientist, who did not want to be identified but who has been involved for years with testing the drugs, says: “If I had cancer, I’d want endostatin or Avastin, or both.”




