Wyss Institute initiates industry partnership to aid brain-targeted drug delivery
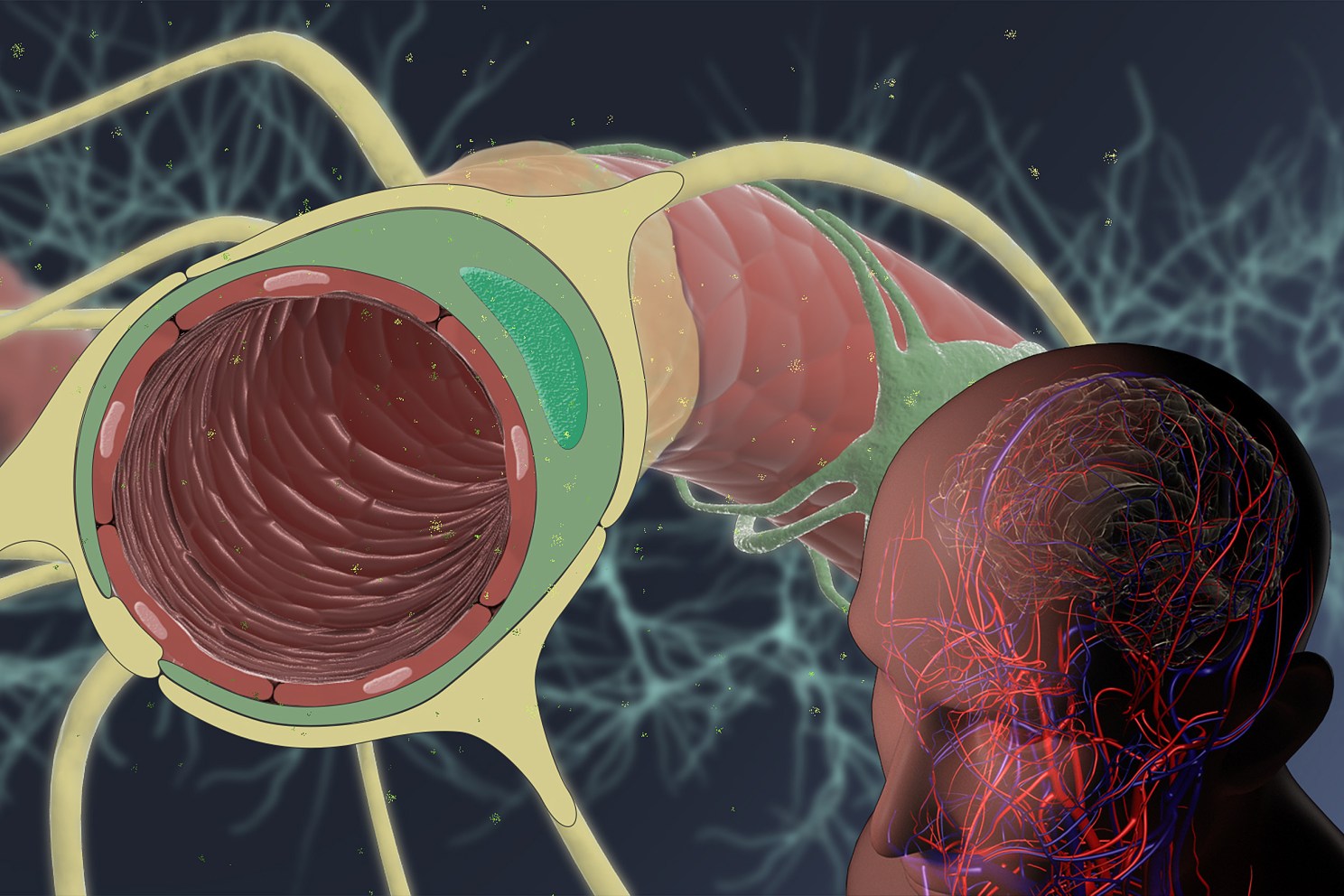
The Wyss Institute initiates a pre-competitive multi-partner industry collaboration to enable more effective delivery of drugs to the brain. Credit: Wyss Institute at Harvard University
The Wyss Institute for Biologically Inspired Engineering at Harvard University announced this week that it is collaborating with multiple industry partners to discover more effective approaches to deliver drugs across the blood-brain barrier (BBB) for the treatment of brain diseases. The main goals of the initiative will be to identify transport target proteins in the BBB and to develop antibody compounds that bind to these targets to facilitate the delivery of future therapeutics to the brain. The pharmaceutical companies Bristol-Myers Squibb Company, U.S.A.; Eisai Inc., Japan; and H. Lundbeck A/S, Denmark; are equally supporting this research effort and will share in the program’s findings to inform their own drug development activities. Through the Massachusetts Life Science Center’s Novel Therapeutics Delivery program, the collaboration was recently awarded a $750,000 grant as additional support.
In additional agreements, the Wyss Institute is collaborating with Cell Signaling Technologies, Inc., U.S.A., to study RNA and protein expression in different tissues in order to identify new BBB transport targets, and with Lundbeck and FairJourney Biologics, a Portugal-based antibody discovery company, to co-develop antibody compounds that would shuttle drugs to the brain via known and novel targets identified in the broader program.
The complementary research agreements, constituting a unique collaboration model for the Wyss Institute, were set in place by Harvard’s Office of Technology Development (OTD).
For the treatment of neurological and neurodegenerative disorders, brain tumors, and many other diseases, transporting therapeutics across the BBB remains a formidable challenge. The capillary vessels, and associated non-neuronal and neuronal cells contacting them on the brain side of the BBB form a highly selective security system that allows nutrients and oxygen to enter the brain from the blood circulation, and waste products including carbon dioxide to be eliminated from the brain. The BBB also actively blocks the entry of pathogens, neurotoxic substances, and most drugs into the brain, including small molecule, antibody, and anti-sense oligonucleotide therapeutics. On average, less than 1% of a drug enters the brain from the bloodstream. The impact of this poor drug transport across the BBB is enormous. For example, in the past decade alone, more than 200 clinical trials of drug candidates for Alzheimer’s disease alone have failed, partly due to poor transport across the BBB resulting in failure to achieve therapeutic drug concentrations in the brain.
The novel pre-competitive initiative began in discussions between James Gorman, principal investigator of the Wyss Institute’s Brain Targeting Program, and Richard Hargreaves, senior vice president and head, Neuroscience Thematic Reseach Center, Bristol-Myers Squibb. “To more effectively treat brain diseases, it is critically important to find better strategies to transport drugs into the brain. However, the complexity of this problem makes it challenging for individual research institutions or companies to solve on their own,” said Hargreaves. “We strongly believe in this type of collaboration to develop tools for drug development and delivery. This collaboration offers a compelling opportunity to discover new approaches for drug delivery to the brain.”
[gz_article_embed type=”embed” layout=”default” caption=”This%20animation%20explains%20how%20Wyss%20Institute%20researchers%20and%20their%20industry%20partners%20aim%20to%20identify%20novel%20transport%20targets%20and%20shuttle%20compounds%20to%20enable%20more%20effective%20delivery%20of%20drugs%20to%20the%20brain.%20″ credit=”Credit%3A%20Wyss%20Institute%20at%20Harvard%20University” ratio=”16-9″]https://www.youtube.com/watch?v=utAEbvlmL58[/gz_article_embed]
The collaboration aims to identify new shuttle target proteins that are highly enriched within brain microvascular endothelial cells that line the miles of BBB microvessels but that are relatively scarce or absent in microvessels in other organs. To do this, the researchers will employ comparative proteomic and transcriptomic approaches and a bioinformatic brain delivery target discovery platform. The identification of shuttle target proteins that are highly enriched in the BBB and able to shuttle drug cargoes across it is critical for improving drug delivery to the brain, and for avoiding side effects and loss of drug due to binding of the shuttle to any of its target protein present in other organs.
To enable more effective strategies to deliver therapeutics to the brain, the Wyss Institute has developed new computational approaches to collect, analyze, and integrate data on potential delivery targets. “When we started searching for new shuttle targets in the BBB, along with compelling shuttle compounds binding to them, we found that the available human data on BBB target expression is quite limited. To address this problem, we launched a multidisciplinary effort at the Wyss Institute using innovative collection, bioinformatic, and target assessment methodology to gather data from human BBB samples and rank potential targets using novel approaches,” said Gorman who, together with Wyss Founding Director Donald Ingber leads the Wyss Institute’s Brain Targeting Program and initiated the new collaborative initiative with the Wyss’ industry partners.
Under the collaboration, Wyss Institute researchers will further investigate target shuttle proteins emerging from this data-driven approach for their potential to transport drug-like cargo molecules, using the Wyss Institute’s antibody shuttle discovery platform that seeks to integrate both human in vitro and humanized in vivo models. The Wyss team is developing humanized mice in which the mouse shuttle target proteins in the BBB have been replaced by the analogous human proteins. In parallel, they are developing human in vitro BBB models that recapitulate drug delivery, some of which could be conducted under dynamic fluid flow, similar to the conditions found in the brain vasculature in vivo. By assessing correlation of the in vitro and in vivo models, the team will select the best assays to identify and rank shuttle proteins that can efficiently transport drugs into the human brain.
“The Wyss Institute’s interdisciplinary, cross-organizational collaboration model brings diverse key capabilities to bear on this extremely difficult problem of targeting therapies to the brain. Our industry partners contribute highly valuable expertise in this challenging field, our clinical collaborators provide diverse human tissue samples, and our internal team provides advanced technical capabilities and a truly unique bioconvergence approach to solve high-risk problems. Through this ambitious effort, we hope to develop new delivery approaches to help treat brain diseases for which there currently are no effective therapies,” said Ingber, who also is the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children’s Hospital, and Professor of Bioengineering at the Harvard John A. Paulson School of Engineering and Applied Sciences.
Separately, to generate antibodies with pharmaceutical quality against multiple shuttle target proteins, Wyss researchers will collaborate with scientists at FairJourney Biologics and Lundbeck. FairJourney Biologics will use its expertise in the discovery of unique antibody formats to develop antibody shuttles that bind to two of the program’s high-priority target proteins. Lundbeck and the Wyss Institute will co-develop antibody shuttles binding to a third high-priority target protein. Through these collaborations, the Wyss Institute aims to generate validated BBB delivery shuttles that will be available for licensing from Harvard.
The $750,000 grant from the Massachusetts Life Sciences Center (MLSC) Novel Therapeutics Delivery program lends additional support to this BBB research consortium. The MLSC program aims to foster in Massachusetts the development of novel technologies and techniques for the delivery of existing or innovative therapies at the intersection of engineering, biology, chemistry, and medicine.