New Alzheimer’s study suggests genetic cause of specific form of disease
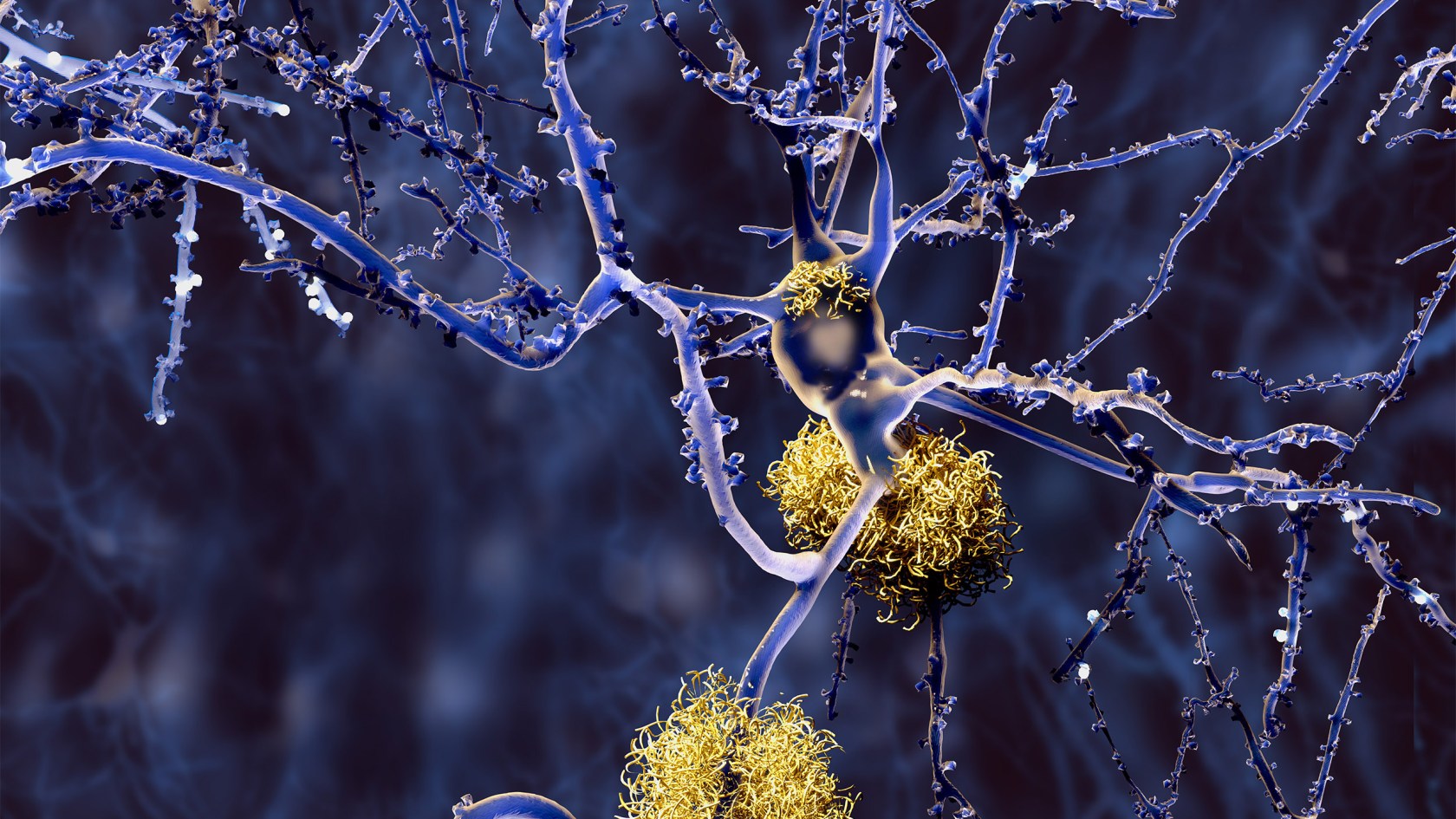
Neurons with amyloid plaques.
Findings eventually could pave way to earlier diagnosis, treatment, and affect search for new therapies
A recent study published in Nature Medicine offers evidence that genetics may be a direct cause of a specific form of Alzheimer’s disease and not merely a risk factor. While most patients currently do not have a clearly identified cause of this devastating illness, researchers found that people with two copies of the gene variant APOE4 are at extremely high risk of developing Alzheimer’s. The finding led them to recommend a new designation that takes this into account, which could lead to up to a fifth of Alzheimer’s patients being classified as having a genetically caused form of the disease. The shift eventually could lead to earlier diagnosis and treatment and affect the search for therapies. Reisa Sperling, a neurologist at Mass General Brigham and an author of the study, explains the importance of the findings. This interview has been edited for clarity and length.
Your study highlights a new, clearly identified genetic component to Alzheimer’s disease worthy of a new designation. Could you explain why that’s significant?
Designating this form of Alzheimer’s disease means a group of people who are extremely likely — I won’t say absolutely, but extremely likely — to develop Alzheimer’s could be treated earlier. This could really have an impact on preventing dementia.
The second thing is there’s been an ongoing debate about whether Alzheimer’s disease has anything to do with amyloid plaques or not. And in this group, they begin to have buildup of amyloid plaques and tau tangles in their late 50s and early 60s, and the likelihood that they will develop symptoms of Alzheimer’s disease is extremely high. So it creates another link in our understanding of the disease process.
And finally, this is a bridge between the rare forms of genetically determined Alzheimer’s disease that are 100 percent penetrant and often affect people in their 40s and 50s. Those cases are often considered such a rarity that they’re not representative of Alzheimer’s disease. So people with two copies of APOE4 are a bit in the middle. This new study really suggests that their biomarkers are similar to what we see in these rare autosomal dominant diseases, and over 90 percent will develop Alzheimer’s pathology in their brains. It links the rare genetic forms of Alzheimer’s to what we call sporadic late-onset Alzheimer’s disease.
Part of this new classification would also make this type of Alzheimer’s one of the most common genetic disorders in the world. Are there benefits to having it classified that way?
I don’t know that I’m the best person to opine on that, but I certainly think there may be important reasons. For example, eventually getting insurance coverage for individuals who are below the age of 65 and need rapid evaluation and treatment for Alzheimer’s disease. Alzheimer’s disease often doesn’t get diagnosed in these individuals because people think they’re too young. Additionally, they may not have insurance coverage for all of the medications required for treatment.
I do think it is important that this is recognized as one of the more common genetic links to Alzheimer’s disease and leads the way to one day being able to treat people who have a strong family history and genetic predisposition. Then we can really think about being aggressive and treating patients early.
“Somehow, we have to turn these findings to — instead of being scary for people — being a sense of hope.”
Reisa Sperling

We’ve known for a long time that there is a genetic component to Alzheimer’s disease. Is this one of the first studies to show such a specific genetic link?
No. As I mentioned there are these rare genes that we’ve known for more than 20 years that are very specific and cause Alzheimer’s disease at a much younger age. But this data really suggests that people who have two copies of this particular allele, APOE4, have such a high likelihood of developing Alzheimer’s disease.
So it’s not the first genetic link, but it is the first large study that convincingly says having two copies of this gene really increases the likelihood you will have Alzheimer’s disease. And it’s a more common gene; these other known genes are very rare. But with APOE4, it’s estimated that up to 15 percent of Alzheimer’s patients carry two copies of these alleles (although I will say that estimate is a little different across studies). It is much more common than these very rare autosomal dominant forms.
How common is it in the general population to have two copies of that gene?
Estimated, about 2 percent of the population, so it’s not that common. People having at least one copy of APOE4 is fairly common. Depending on which part of the world you’re from, that can be up to 25 percent. But having two copies is still pretty rare.
There is still so much that we don’t know about Alzheimer’s, but it does seem to be fueled by both genetic and environmental factors. In what ways does this research help push our understanding of the disease overall?
That’s a great question. And for me, this research really does provide support to both camps. One, the likelihood that people with these genes will develop amyloid plaque by the time they’re age 65 is somewhere between 75 and 95 percent. To me that suggests that it is genetically driven.
But there is a variability in the range of when people develop symptoms. And that suggests that there might be environmental or lifestyle factors that can make people’s brains more resilient, or conversely, more vulnerable. This research really supports both ideas that genetics is a major driver in Alzheimer’s disease, but you can modulate your risk of showing symptoms.
Would it be beneficial for people to know early on if they are carriers of these genes?
At this moment, I do not recommend that people who don’t have symptoms get genetic testing or blood-based biomarker testing. I hope that recommendation will change greatly over the next few years.
There are large-scale clinical trials, including the one I run. We’re recruiting people who have evidence of amyloid buildup, but don’t yet have symptoms, and we’re recruiting a lot of people with a family history and have copies of APOE4. If that study and other studies like it succeed in treating people before they have symptoms, then I would recommend testing and trying to get treatment as soon as possible.
But we don’t have that available right now, and I just think we don’t yet know what to do with that information before people have symptoms.
If this new classification did occur, what areas of further research would you be most excited to pursue?
Number one for me is we need to be able to offer treatments to those patients. Right now, there’s actually a black-box warning on some currently approved Alzheimer’s treatments that cautions treating people who have two copies of APOE4 because the risk of side effects is so great. I want to redouble my efforts to make sure we can offer disease-modifying medications in a safe way.
Number two is about the environment. I’m quite interested in what it is that modulates whether people get symptoms sooner rather than later, with this buildup of amyloid that’s genetically determined. How do we understand what factors were protective? That’s a very important area of research to help us understand what can modulate people’s risk of symptoms in the setting of a very strong genetic predisposition.
We talk about this in the study, but I think it’s also important to mention that these studies mostly observed white majority populations. And one of the things we desperately need to know is whether these findings are also true in more ethnic and racially diverse populations. There is some evidence that APOE4 might have a slightly different effect on amyloid in populations who come from communities of color.
Similarly, there are slight differences in the sex effects: Women APOE4 carriers have more likelihood of developing symptoms. I think it’s really important to get more information on representative populations, especially from communities of color, and really help us develop treatments that will work best for everybody.
For people who have Alzheimer’s or loved ones with Alzheimer’s, how do these findings offer hope or shed light on the disease?
This is another tool to be able to find people who have Alzheimer’s disease at an earlier stage and treat them earlier. My dad and my grandfather died of this disease, and I’m a clinical neurologist. When I see people with symptoms, I think this is helping us learn about the underlying causes and will help us in accelerating to find good treatments.
I think it will both help the next generation of people who are likely to develop Alzheimer’s disease, but it will also help us treat people who already have Alzheimer’s disease symptoms because every little bit of information helps us develop better treatments for all.
I really hope this research doesn’t have the effect of just scaring people. I hope it will instead say, “These are important clues so that we can treat people earlier and hopefully prevent dementia.”
Somehow, we have to turn these findings to — instead of being scary for people — being a sense of hope. I hope this means we will be able to find people and treat them before they develop symptoms.
Get the best of the Gazette delivered to your inbox
By subscribing to this newsletter you’re agreeing to our privacy policy