Evolutionary biology research team members: Amar Sarkar (from left), Rachel Carmody, and Cameron McInroy.
Jon Chase/Harvard Staff Photographer
We’re social beings. So are microbes.
When we pick up our neighbors’ bugs, we get the good as well as the bad and the ugly
The COVID-19 pandemic reminded us that social interactions transmit pathogens. But do humans spread “good” bugs, too?
Very much so, say a team of biologists who are probing the links between the microbiome and health. In a recent perspective piece in Cell, they explain why the “social microbiome” — the microbial metacommunity associated with a social network of humans or other animals — deserves attention. The researchers pointed out that microbial sharing could play a role in individuals’ susceptibility to, and resilience against, both communicable and non-communicable diseases.
“When we think of factors that affect the microbiome, diet and antibiotics come to mind most readily,” said lead author Amar Sarkar, a Harvard Griffin Graduate School of Arts and Sciences student in the Department of Human Evolutionary Biology. “But the fact that our social interactions also affect the microbiome is less well appreciated.”
The researchers first introduced the concept of the social microbiome in a 2020 article in Nature Ecology and Evolution. There, Sarkar and co-authors suggested that the social microbiome could be viewed as a sort of archipelago, each of us being an “island” that hosts a distinct set of microbes. We share our microbes with one another, similar to how birds or insects might disperse across islands. Their latest analysis looks at why that framework matters.
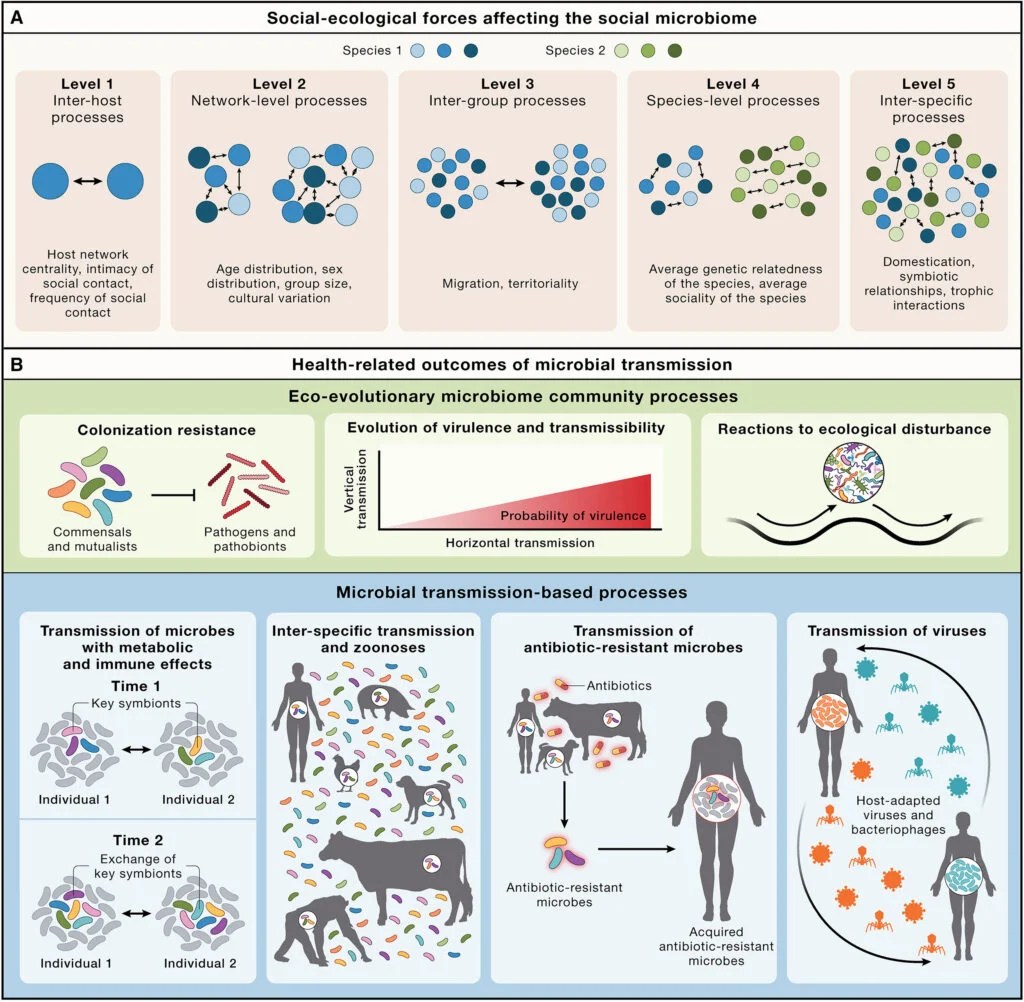
Processes at different social-ecological scales influence the social microbiome, and health-relevant processes are affected by the social transmission of microbes.
Credit: Cell
Focusing on the gut microbiome and its implications for overall health, the researchers offer an analysis of social transmission of microbes at five levels, each increasing in ecological scale. They range from host-to-host interactions that spread through direct contact between individuals, like hugging and touching, all the way up to inter-species microbial mingling — think our close ties with our pets.
Social microbial transmission can take place from parent to infant early in life, and through direct and indirect contact with others throughout life. This is why household co-habitation leads to substantial microbial strain-sharing between family members. The researchers cite studies showing that individuals within a household shared a significant proportion of their gut microbial strains, and that villages could be distinguished on the basis of their social microbiomes.
The importance of microbial connections in human health outcomes is an area with enormous potential for discovery, according to the researchers. Many chronic conditions historically considered to be “noncommunicable” — including metabolic diseases, cardiovascular disease, autoimmune disorders, and certain cancers — are now being evaluated for their microbial causes and correlations. In many cases, the microbes that shape susceptibility to diseases, or responses to treatment, have been shown to be socially transmissible.
“If microbes contributing to disease can be transmitted between individuals, some noncommunicable conditions may in fact have a communicable component,” said Rachel Carmody, associate professor in Human Evolutionary Biology and co-senior author of the work. “While that may be a potentially unsettling thought, socially transmitted microbes may help protect against these conditions, too.” For example, studies have shown that mice living in the same cage can transmit microbes that increase resilience against colitis or improve their responsiveness to cancer therapy.
“We observe instances where social microbial transmission protects against disease, and instances where it promotes disease, but further work to probe these effects in humans is necessary,” said co-author Cameron McInroy ’22, a teaching fellow in the same department. “Key challenges lie in pinpointing the microbes and transmission contexts controlling these risk-decreasing and risk-increasing effects, understanding how they work, and ultimately harnessing them for our benefit.”
The researchers considered the impact of the social microbiome for antibiotic use. Antibiotic exposures can be thought of as disturbances to the microbiome ecosystem, similar to how fires disturb forest ecosystems. Altering the gut microbiome, sometimes for extended periods, can increase the risk of acute infections such as Clostridioides difficile. Social interactions after antibiotic exposure could help the microbiome recover from these antibiotic-induced disturbances.
The researchers also looked at the global problem of antibiotic-resistant microbes arising and spreading due to exploitation of antibiotic drugs. The transmission of such microbes may have more social components than are currently appreciated, the researchers say. For example, individuals who share a household might acquire antibiotic-resistant microbes from each other if some members are under prolonged antibiotic treatment. What’s more, cultures, societies, and countries differ in their usage of antibiotics, they say, creating “culture-dependent transmission landscapes” for antibiotic-resistant microbes.
Evolutionary biologists have long proposed that important benefits of group living — such as protection from predation, improved territorial defense, and social learning — come at the price of higher pathogen transmission. In their article, the researchers raise the prospect that some social behaviors may also have evolved to transmit beneficial bacteria among individuals.
“Social interactions can provide conduits for pathogen transmission, but beneficial microbes are also known to be transmitted through these interactions,” said co-senior author Andrew Moeller of Princeton University. “It may be that, in some contexts, the benefits provided by socially transmitted mutualists outweigh the costs incurred by socially transmitted pathogens.”
The authors of “Microbial transmission in the social microbiome and host health and disease” were supported in part by the National Science Foundation, National Institutes of Health, National Cancer Institute, and National Institute of General Medical Sciences.






