The i3 Center will increase tumor-specific activities of cytotoxic T cells, by boosting different stages of the normal process by which T cells develop, and acquire anti-cancer activity.
Shutterstock/SciePro
i3 Center formed for advancing cancer immunotherapy
Will develop new biomaterials-based approaches to enable anti-cancer immune-therapies for therapy-resistant cancers
A new NIH-funded center, which will bring together cancer immunologists and biological engineers to develop new approaches for therapy-resistant cancers, was announced today.
Harvard University’s Wyss Institute of Biologically Inspired Engineering and its collaborating institutions, the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), Dana-Farber Cancer Institute (Dana-Farber), and Harvard’s Department of Stem Cell and Regenerative Biology, announced the formation of the Immuno-Engineering to Improve Immunotherapy (i3) Center. The cross-institutional and cross-disciplinary i3 Center includes world-leading researchers in the cancer immunology and bioengineering fields and will create biomaterials-based approaches to enable anti-cancer immuno-therapy in settings where it currently is limited, such as in myeloid malignancies and solid tumors.
The Harvard i3 Center is part of NIH’s Cancer Moonshot initiative that was formed to accelerate cancer research to make more therapies available to more patients, while also improving the ability to prevent cancer and detect it at an early stage.
“We aim to develop new technologies that induce robust anti-cancer T cell immunity, and we also hope that the i3 Center’s highly cross-disciplinary and cross-fertilizing mechanisms will provide a center of gravity for many future efforts in the immuno-therapy space across and beyond our collaborating institutions,” said Wyss Institute Founding Core Faculty member David Mooney, one of the two principal investigators (PIs) of the i3 Center.
Mooney also is the Robert P. Pinkas Family Professor of Bioengineering at SEAS and leads the Wyss Institute’s broader Immuno-Materials Initiative. His team has developed a number of strategies that use immune-modulating biomaterials to trigger and enhance T cell-mediated immune responses against tumors. Most notably, together with clinical collaborators, they succeeded in creating the first implantable vaccine ever to eliminate melanoma tumors in mice, which the Wyss Institute and Dana-Farber are investigating in an ongoing Phase I clinical trial at the Dana-Farber.
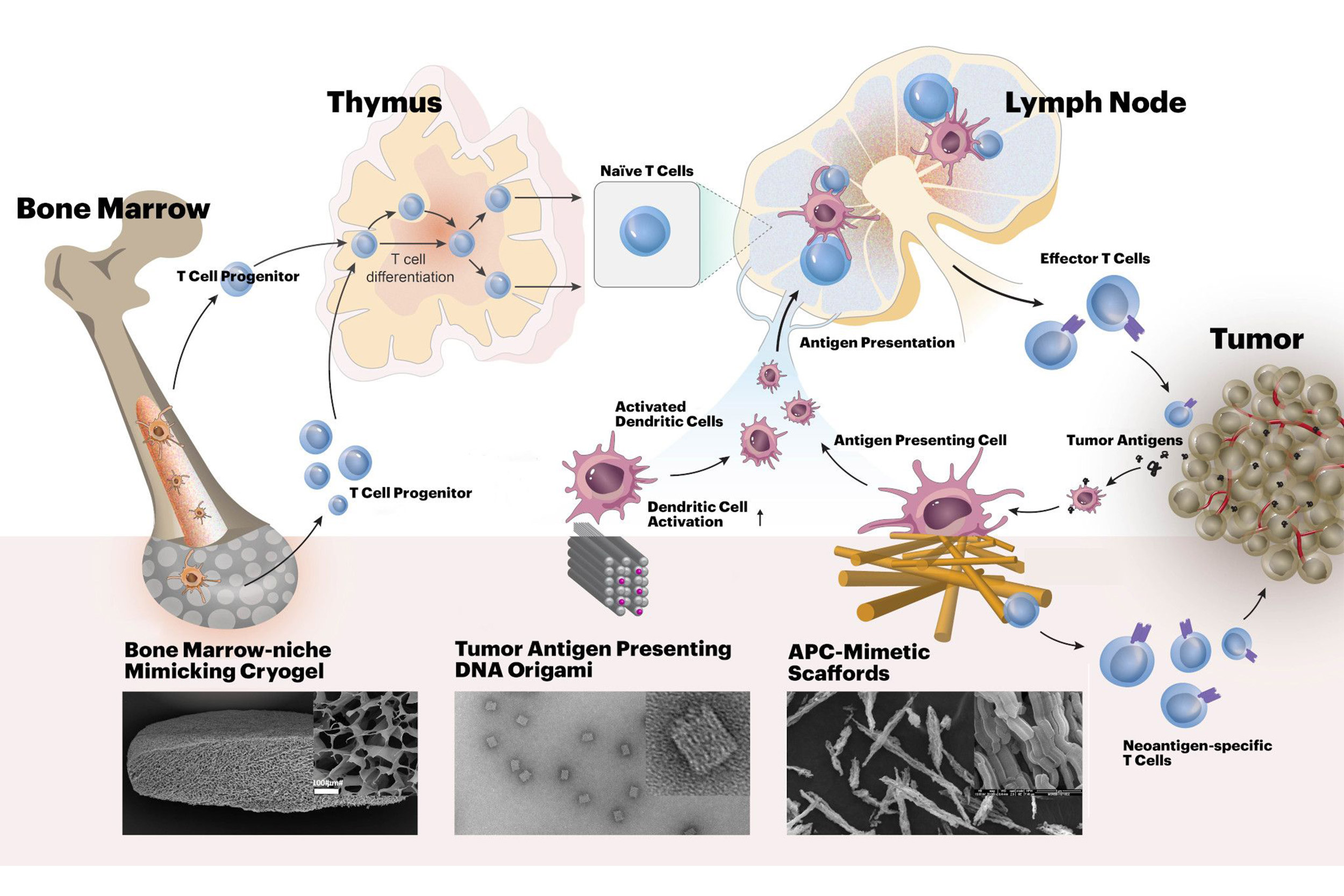
Harvard’s i3 Center will develop new biomaterials-based approaches for cancer immunotherapy. The materials will enhance tumor-specific activities of cytotoxic T cells, acting at different stages of their development.
Credit: David Zhang/Wyss Institute at Harvard University
Steven Hodi Jr., the i3 Center’s other PI, and director of Melanoma Center and the Center for Immuno-Oncology at Dana-Farber, and professor of medicine at Harvard Medical School (HMS), is leading the clinical cancer vaccine trial. He has been at the forefront of developing cancer immunotherapies using “immune checkpoint inhibitors,” a class of drugs able to re-activate tumor-destroying T cells that are muted in the tumor microenvironment. “The funding for this center provides a unique opportunity to unite key investigators for translating fundamental advancements in immunology and biomedical engineering into highly synergistic approaches to improve the treatments for cancer patients,” said Hod
Using both in vivo and ex vivo biomaterials-based approaches, the i3 Center aims to boost tumor-specific activities of cytotoxic T cells, by boosting different stages of the normal process by which T cells develop, and acquire anti-cancer activity. T cells’ normal development starts in the bone marrow where hematopoietic stem cells generate T cell progenitor cells. These migrate to the thymus to differentiate into naïve T cells, which then travel further to lymph nodes. There, they encounter cancer-derived antigens presented to them by specialized antigen-presenting cells (APCs) that can activate T cells to recognize and eliminate cancer cells.
In relation to “adoptive T cell” therapies in which T cells are given to patients to fight their cancers, one team at the i3 Center will be led by Dana-Farber researchers Catherine J. Wu and Jerome Ritz, who along with Mooney, will develop and test biomaterials that can better mimic normal APCs in activating and directing the function of patient-derived T cells outside the human body, prior to their transplantation. Wu is chief of the Division of Stem Cell Transplantation and Cellular Therapies, and Ritz is executive director of the Connell and O’Reilly Families Cell Manipulation Core Facility at Dana-Farber.
“We need to make efforts to enhance the ability of the immune system to recognize tumor cells. One direction my laboratory is taking makes use of innovative biomaterials to help us to efficiently expand polyclonal tumor-specific functionally-effective T cells ex vivo in a way that can be readily translated to the clinical setting. In our studies, we are currently focusing on melanoma and acute myeloid leukemia,” said Wu, whose research interests include understanding the basis of effective human anti-tumor responses, including the identification and targeting of the tumor-specific antigens.
A second project explores the use of DNA origami, biocompatible nanostructures composed of DNA, to create cancer vaccines. DNA origami could provide significant advantages in presenting tumor-specific antigens and immune-enhancing adjuvants to APCs because the concentrations, ratios, and geometries of all components can be modulated with nano-scale precision to determine configurations that are more effective than other vaccination strategies. The project will be run by Wyss Institute Core Faculty member William Shih, Derin Keskin, lead immunologist at Dana-Farber’s Translational Immunogenomics Lab, and Mooney.
In a third project, David Scadden, professor at Harvard’s Department of Stem Cell and Regenerative Biology, will collaborate with Mooney to build on their previous work. They will engineer biomaterials that recreate key features of the normal hematopoietic stem cell niche in the bone marrow. Such implantable biomaterials could help rapidly amplify T cell progenitor cells, and enhance T cell-mediated anti-cancer immunity. Scadden also is the Gerald and Darlene Jordan Professor of Medicine at Harvard University, and co-director of the Harvard Stem Cell Institute.
The i3 Center’s investigators anticipate that it will stimulate additional cross-disciplinary concepts and research, due to the culture of continuous interactions, sharing of findings, data and samples between all investigators, as well strong biostatistical expertise provided by Donna Neuberg, a senior biostatistician broadly involved with exploring immune-modulating cancer interventions at the Dana-Farber.
“This new i3 Center for cancer immunotherapy innovation really embodies how the Wyss Institute with its unparalleled capabilities in bioengineering and serving as a site for multidisciplinary collaboration, and can liaise with clinicians and researchers at our collaborating institutions to confront major medical problems and bring about transformative change,” said Wyss Founding Director Donald Ingber. He is also the Judah Folkman Professor of Vascular Biology at HMS and the Vascular Biology Program at Boston Children’s Hospital, and Professor of Bioengineering at SEAS.





