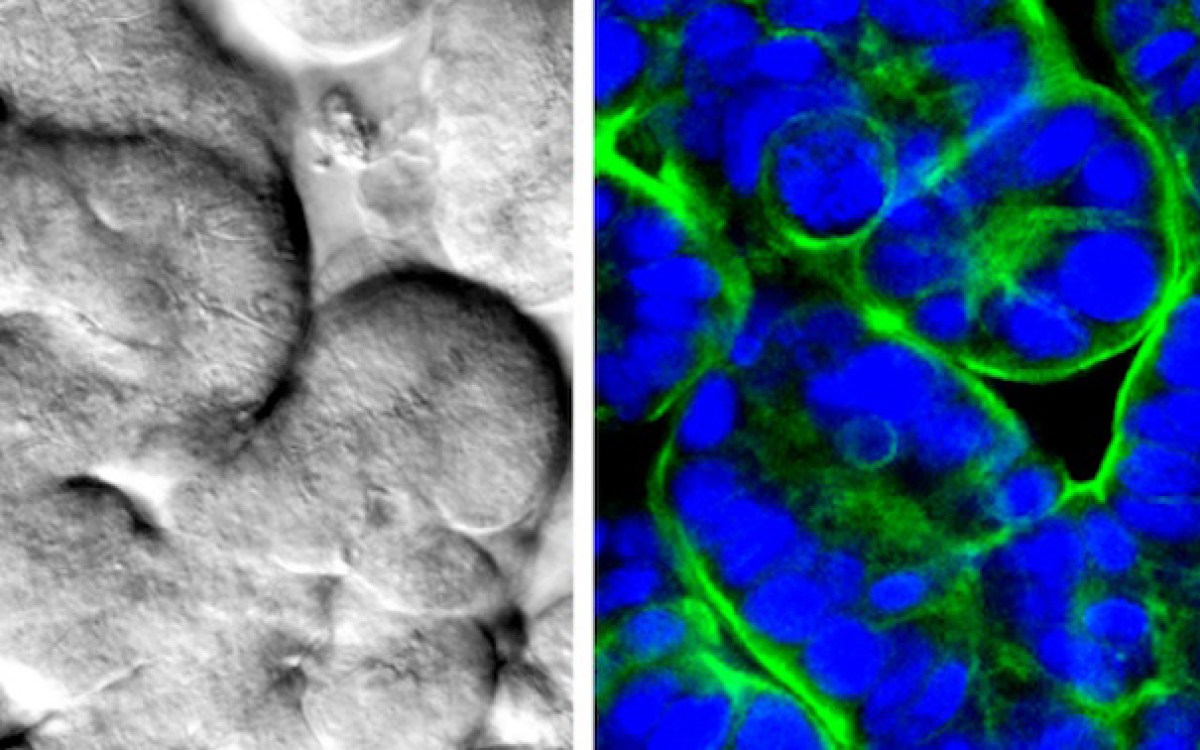
“When cancer patients receive radiation treatment for a specific organ, other organs are also affected, mostly the bone marrow, the lung, and the gut,” says first author Sasan Jalili-Firoozinezhad. Shown are normal, undamaged villi-like protrusions in the small intestine.
Credit: Wyss Institute at Harvard University
Study of radiation exposure in human gut offers hope
Organ-on-a-chip is starting point for radioprotective drugs for cancer patients
Chernobyl. Three Mile Island. Fukushima. Accidents at nuclear power plants can cause massive destruction and expose workers and civilians to dangerous levels of radiation that lead to cancerous genetic mutations and death. While the total number of people affected by nuclear incidents is small, every year millions of cancer patients around the world receive radiation therapy which, while lower-dose, can still cause harmful cumulative side effects.
Because exposing healthy people to radiation for clinical trials would be unethical, efforts to identify drugs that can mitigate the effects of radiation exposure have been limited to animal studies, which are notoriously poor predictors of how a given drug will behave in humans. Now, researchers from the Wyss Institute for Biologically Inspired Engineering at Harvard University, Instituto Superior Técnico (IST, Portugal), Boston Children’s Hospital, and Harvard Medical School (HMS) have published a study using an organ-on-a-chip model of the human gut that reveals that intestinal-blood-vessel cells may play an important part in radiation-induced intestinal injury. It also confirms that a potential radioprotective drug, dimethyloxaloylglycine (DMOG), suppresses the intestine’s responses to radiation injury. The results are reported in Cell Death & Disease.
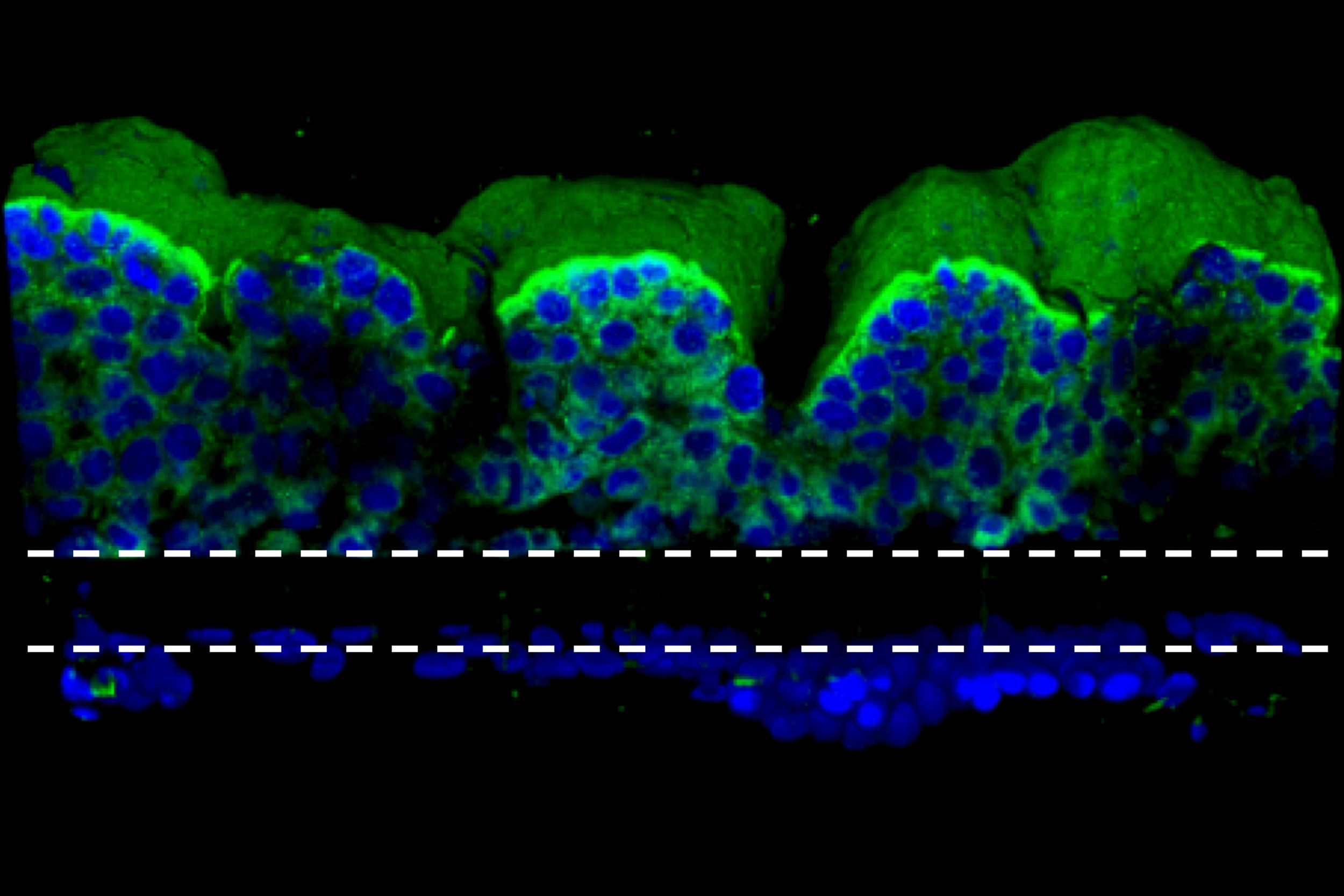
“When cancer patients receive radiation treatment for a specific organ, other organs are also affected, mostly the bone marrow, the lung, and the gut,” says first author Sasan Jalili Firoozinezhad, who is a graduate student at both the Wyss Institute and the Institute for Bioengineering and Biosciences at IST. “Radiation in the gut causes the microvilli projections of the epithelial cells to retract, leading to poor nutrient absorption, leakage through the normally tight intestinal barrier, and destruction of the beneficial microbiome. We wanted to see if we could replicate that response in our gut chip, and then reverse it with DMOG.”
The gut chip is a microfluidic device made of a clear, flexible polymer that contains two parallel microchannels separated by a porous extracellular matrix membrane. One channel is coated with human intestinal epithelial cells, the other with human endothelial cells that mimic the blood vessel wall. Cell culture medium is perfused through both channels, and suction is applied to side chambers within the chip at regular intervals to cyclically stretch the tissues, mimicking the peristalsis-like motions that move food through the intestine. Under these conditions the epithelial cells spontaneously form intestinal villus-like structures and surface microvilli that increase the cells’ surface area for nutrient exchange, much as they do in living intestines.
When the researchers exposed the gut chip to a radiation dose of 8 gray, which is known to cause gastrointestinal effects in humans, they observed increases in several markers of cell damage in both the endothelial and epithelial cells: apoptosis (or cell death), generation of reactive oxygen species (ROS, or free radicals), double-stranded DNA breaks, degradation of lipids in the cell membrane, and loss of microvilli structure, as well as disruption of the junctions between neighboring cells and of the mucous membrane that protects the intestinal wall from bacteria and toxins.
Interestingly, the endothelial cells in the blood-vessel channel had a stronger response to radiation than the epithelial cells in the intestinal channel in terms of ROS generation, lipid degradation, and DNA fragmentation. Furthermore, the endothelial cells experienced peak apoptosis levels about 24 hours after exposure, while epithelial cells were hardest hit at 48 hours, suggesting that the endothelium is more sensitive to radiation.
When the scientists repeated the experiment on a chip that contained only epithelial cells, the irradiated cells did not have the typical response to radiation: Their microvilli were not blunted, their mucosal barrier was not disrupted, their cell barriers remained functional, and they had lower ROS generation. This surprising result suggests that endothelial cells play a key role in the gastrointestinal damage observed in radiation injury.
“This finding helps explain why other models of the human gut that don’t include endothelial cells generally fail to mimic the gut’s response to radiation injury,” says Oren Levy, co-author of the paper and staff scientist at the Wyss Institute. “More studies are needed to confirm the link between endothelial and epithelial cell responses, but we think that ROS generated by endothelial cell damage will prove to be the driving force behind epithelial cell damage, and this could serve as a target for future anti-radiation therapeutics.”
The team then exposed the gut chip to DMOG, which has been shown to prevent radiation injury in rats by increasing the production of the protective proteins HIF-1α and HIF-2α prior to administering radiation. They observed that DMOG pretreatment significantly reduced apoptosis, ROS generation, and lipid degradation in both epithelial and endothelial cells, intestinal permeability, and microvillus injury of intestinal epithelial cells, a result that has never been demonstrated in human tissues before.
“Now that we have successfully tested a potential drug candidate in a human organ system, our goal is to use this chip to identify new radioprotective drugs by physiologically mimicking radiation damage in the gut,” says Jalili Firoozinezhad.
While this study used a line of infinitely replicating Caco-2 human intestine cells, a newer iteration of the gut chip uses primary human intestinal cells taken directly from patients. “The grand vision for the future of this technology is to link different organ chips into a fully personalized body-on-chips model, where we’d be able to take cells from a patient and test which medicines will best protect all their organs from radiation, either higher doses from nuclear events or lower doses from off-target cancer treatment,” says Levy.
This research was supported by the U.S. Food and Drug Administration, Harvard’s Wyss Institute for Biologically Inspired Engineering, and the Fundaçȁo para a Ciȇncia e a Tecnologia Portugal.
To read the full article, visit the Wyss website.