Lessons from cancer research
Learning to make the best of blood vessels
Rakesh Jain looks at tumors from an engineer’s perspective. The view he gets has led to some startling results.

For example, Jain has learned how to artificially grow networks of blood vessels that someday may be used for regenerating tissues to replace those damaged by illness, trauma, or genetic bad luck. He can also explain how some of the newest cancer drugs don’t work the way everyone thinks they do.
Raised in India, Jain, now a professor of tumor biology at Harvard Medical School, spent most of his career as a chemical engineer. He was working on a computer model of pollutants in the Delaware River as a graduate student at the University of Delaware when his adviser introduced him to researchers at the National Cancer Institute. They were puzzled by the fact that drugs injected into breast, prostate, and other tumors quickly pass out again without much effect on the malignancy.
“I thought, ‘This is an engineer’s dream,’” Jain recalls. “Tumors are like chemical reactors, vats where you try to control what goes in and what comes out. The flow of a river is similar to the river of life, blood flow. That experience changed my life. I decided to apply everything I knew about chemical engineering to solve the problems of cancer biology.”
After serving on the chemical engineering faculties at Columbia University and Carnegie-Mellon University, Jain became the Andrew Werk Cook Professor of Tumor Biology at Harvard and director of the Steele Laboratory of Tumor Biology in the Department of Radiation Oncology at Massachusetts General Hospital in Boston. In the past 10 months, Jain and his colleagues have published six scientific reports demonstrating how an engineering perspective can contribute to understanding cancer and tissue engineering.
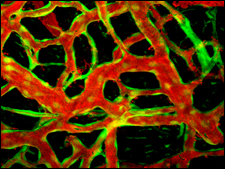
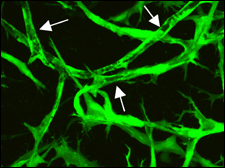
His latest paper, published in the March 11 issue of the British scientific journal Nature, describes how it is possible to artificially grow a stable network of blood vessels using cells from a human umbilical cord. Such a network could nourish replacement tissues or organs, which themselves might be constructed from adult or embryonic stem cells.
Until now, laboratory-grown blood vessels didn’t last very long, not long enough to use for replacements that must last a lifetime. To solve the problem, a Jain team, led by Naoto Koike, a research fellow at Mass General, mixed human cells from the lining and muscular outer coating of umbilical cord veins. The combo was nurtured in culture for about a day, then Koike and his crew transferred the cells to the brains of mice where they started to form long, branching tubes.
“The implants survived, grew, and connected beautifully with the mice’s own system of blood vessels,” Jain explains. “These networks have lasted for a year, about half the lifetime of a mouse.”
Window into the brain
How does anyone know what’s going on physically in a mouse’s brain? Jain engineered a solution to that problem years ago. He developed transparent compartments, implanted windows that allow him to look at blood vessels and tumors as they grow in the brain. Jain and his colleagues have used such windows to watch biology and disease work in the livers, breasts, and other organs of animals.
“These systems are a tool just like the computer models I made of what was happening in the Delaware River,” Jain comments. He believes that the engineered blood vessels will be useful to other research teams working at Mass General and elsewhere to fashion replacements for skin, muscle, kidneys, bladders, and even hearts.
Jain even sees the possibility of growing new blood vessels from a person’s own cells. That would involve isolating stem cell-like precursors of blood vessel linings and outer-surface layers. They would be given a head start in culture dishes, and then implanted in their donors. “It’s a dream, many years away,” he admits, “but clearly within reach. There are people working on it at Harvard and elsewhere.”
Stunting tumor growth
Another study by Jain’s group showed, for the first time, how drugs thought to interfere with development of new tumor blood vessels actually work in cancer patients. These so-called anti-angiogenesis drugs apparently shrink tumors, not by choking off their blood supply, but by improving the delivery of other drugs (chemotherapy) and radiation.

Anti-angiogenesis drugs made headlines around the world when the first ones were developed in the early 1990s. At first, it was thought that these drugs alone would be enough to kill tumors. However, tests on patients revealed that they work best when given in combination with other drugs or radiation.
In February, the U.S. Food and Drug Administration gave approval to the first of the drugs for use with chemotherapy to treat colon and rectal cancers that has spread to other parts of the body. The drug, called Avastin (bevacizumab), is made by Genentech.
Also in the same month, Jain’s group published, in the journal Nature Medicine, the results of a study that seems to show that killing off blood vessels in human tumors improves the blood supply instead of choking the tumor to death by cutting off its blood supply. In this study, led by Christopher Willett, a professor of radiation oncology, six patients with rectal cancer received Avastin. Two weeks later, the number of blood vessels feeding the tumors had been cut in half; but the tumors continued to get the same amount of nutrients.
What had happened? Jain thinks that Avastin quickly pruned immature, inefficient vessels. With them out of the way, the survivors started recruiting the help of support cells they need to form a muscular outer covering. “What was chaotic growth in the beginning gave way to orderly development,” Jain says. “Avastin fortified the tumors’ blood vessels and made them more efficient.”
That’s not bad, Jain insists. The reinforced vessels carry more of the drugs designed to kill tumor cells and the oxygen needed to make radiation therapy effective. To prove that, Willett and his team added chemotherapy and radiation treatment. Six weeks after the end of that combination therapy both the tumors and their blood supply had decreased markedly, as did the chances that the tumors would shed cells that spread cancer throughout the body.
“When we saw this happen in the first patient, I thought it was only luck,” Jain recalls. “But then we saw it in all the other patients. That made me think back to the time when I decided to switch careers and solve the mystery of why most drugs that were going into solid tumors were coming right out. Now, I know it’s because pressure in the tumor was too high to let the drug out of the blood vessels. Drugs like Avastin lower the pressure. That’s a satisfying result from an engineering point of view. From the point of view of treating patients, we’ve learned something valuable from cancer that can be applied to regenerating damaged and diseased tissues.”




