Electric eye under development
Artificial retina nearly in sight
Severely blind people have been able to temporarily see patterns of light with the help of an electric device developed by a Harvard-M.I.T. research team.
Five people with retinitis pigmentosa, the leading cause of inherited blindness, reported seeing spots and lines of light with the help of the device. A sixth person, about to lose her sight to cancer, also saw the light patterns. The retina is a screen of cells at the back of the eye that records light coming through the pupil and converts it to nerve pulses that register as vision at the back of the brain. The artificial retina directly excites these nerve cells with electric probes.
“Many of us believe we can create vision by stimulating optic nerve cells electrically,” says Joseph Rizzo of the Massachusetts Eye and Ear Infirmary, an affiliate of the Harvard Medical School. “So far, we find that it’s possible to induce spots of light in patients who have been severely blind for decades.”
By “we,” Rizzo means himself, John Wyatt of the Massachusetts Institute of Technology’s Research Laboratory of Electronics, and the Boston Veterans Administration Medical Center. The team includes 26 experts in various fields

ranging from surgery to microfabrication. “Some of these people could earn much higher salaries by working in industry, but the possibility of restoring vision to the blind motivates them more deeply than money,” notes Rizzo, who is also an associate professor of ophthalmology at Harvard Medical School. “One expert worked free one day a week, then upped it to two days a week. That was when we had little money, but now we’re able to pay him.”
The research team’s overall goal is to improve the quality of life for the 1.6 million people worldwide who have lost their sight to retinitis pigmentosa and the millions more who are afflicted with macular degeneration, a progressive loss of vision with age. Seeing basic light patterns is a first step toward enabling these sightless people to walk safely in unfamiliar places, such as streets, stores, buses, and trains. After that might come enough vision to recognize faces, even to discern emotional expressions. Finally, it may be possible for the blind to read magnified text.
Those working toward such goals foresee a minute camera mounted on eyeglass frames. The visual scenes in its lens would be converted to electrical patterns by a computer microchip, then radioed to an array of wires ten times thinner than a human hair. The wires would send the same signals into the brain as the sight of a smiling face on a sunlit day.
Signal for sight
Rizzo, Wyatt, and their colleagues are pleased with their results so far, but sobered by tough challenges they see ahead.
In experiments that lasted hours, each of the six people sketched what they “saw” while tiny electric pulses tattooed an extremely sensitive part of one eye. The eye of the sighted person was later removed because of cancer, and close examination of her retina showed no damage. But what would be the result of electric energy and heat impinging on nerves for years and years? At this point, no one can answer that question.
Then there’s the challenge of “biocompatibility,” successfully marrying engineering and biology. Engineering materials must function over a long period without being rejected by the immune system or interfering with natural body functions. Scientists and engineers who work on artificial hearts know the problems well.
A more basic challenge involves cracking the code of signals that pass between the eye and the brain. What combination of brain cells must fire for someone to see a tree, a face, a scowl? “At this point, we feel like we have a piano, but don’t know which keys to hit to create a symphony of sight,” Rizzo muses.
“We have the greatest admiration for the patients who work with us,” he notes. “They willingly accept the heroic risks of people going into their eyes without any immediate benefits.”
The experimenters cut a paper-thin slit into the eyes of their patients, who ranged in age from 30 to 70 years. They slipped a small array of incredibly thin wires into contact with still-functioning nerve cells in the retina. Then they sent in electric signals to create the “sight” of spots and lines. These people were awake during the tests so they could describe and draw what they saw. One big surprise (and disappointment) was that not everyone, including the sighted person, saw the same thing.
“A little more than half the time our patients saw patterns of light that matched the geometric pattern of electric stimulation,” Rizzo says. “However, in some cases, where we expected a single electrode to produce a single spot, the patients saw a cluster of two or three spots. That speaks to our greatest area of ignorance, determining how retinal cells respond to changes in light, color, edges, and movement, then developing electrical signals that mimic this response. Before we do more testing on humans we must develop signal patterns and strengths that are likely to be more successful.”
Going behind the eye
Groups all over the world are attempting to develop ways to make the blind see. There are two companies in the United States, as well as research teams in Australia, Germany, Korea, and Japan trying to make artificial retinas.
A firm called Optobionics in Wheaton, Ill., is already implanting tiny silicon chips, some of which have been in patients’ eyes for more than a year. Second Sight, a Los Angeles firm, also has implanted artificial retinas in patients for varying lengths of time. Optobionics’ device contains thousands of microscopic light detectors that directly activate nerve cells without the additional stimulation of electric currents like those used by the Harvard-M.I.T. team. Alan Chow, one of the inventors, reports that wearers see well enough to recognize faces and details of clothing. But these results, and similar claims from Second Sight, have not been verified by independent sources.
A debate exists about what these patients actually see. People with retina pigmentosa and macular degeneration, the leading cause of blindness in older adults, once had the ability to see. Are they really seeing again or are they remembering or imagining the details they report? Rizzo has formed a subgroup of researchers who are addressing this question.
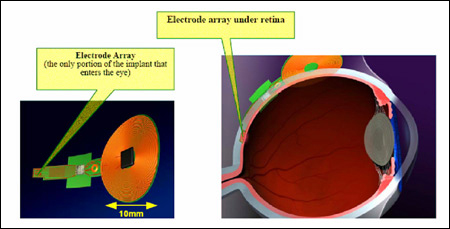
Optobionics places its silicon chip behind the retina, whereas the Rizzo-Wyatt group stimulated the front of it with their electrodes. To help solve the difficult biocompatibility problem, Rizzo says that the next devices they implant will be placed behind the retina. “That will allow us to put 99 percent of the materials, the electric source and the chip that regulates stimulation patterns, outside the eye,” he explains. “The only thing inside would be a flexible chip thinner than a human hair.”
This approach would limit damage from radiation and heat generated by the electric source. It also frees the inventors from having to seal artificial parts from the salty fluid (vitreous humor) that fills the eye between the lens and retina.
With the knowledge and experience his team has gained so far, Rizzo feels confident that the biological and engineering problems will be minimized. “No device will be perfect,” he admits. “What we are striving for is an acceptable balance between imperfections and benefits. This is a long-tern effort, but we feel that an artificial retina will eventually be produced which will improve the quality of life for blind people.”




