Genes found that regulate brain size:
One increases, the other decreases

Two genes that determine brain size have been discovered. One can increase the thinking parts of mice brains, possibly making the rodents smarter. The other is present in people with microcephaly, a genetic disease characterized by a smaller-than-normal brain and head. Such people are mildly retarded.
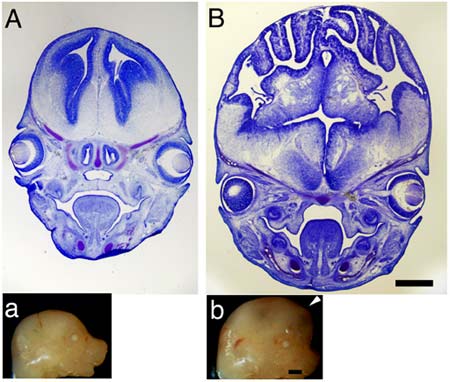
The gene that builds bigger brains, called beta-catenin, was discovered in the laboratory of Christopher A. Walsh, Bullard Professor of Neurology at Harvard Medical School (HMS). Researchers there engineered increased activity into the beta-catenin gene in mice, and the mice brains grew to almost twice the usual size. Not only that, but the cerebral cortex, seat of intelligence and language, became more humanlike.
Walsh and his colleagues, working with C. Geoffrey Woods at St. James University Hospital in Leeds, England, also identified a gene known as ASPM, mutations of which are a major cause of inherited microcephaly. Finding ASPM opens the way to genetic counseling and prenatal diagnoses for families at high risk for the disease. (Microcephaly is more prevalent in Asian nations from Jordan to India, where arranged marriages between relatives are common.)
“We don’t yet know if engineering the ASPM or beta-catenin genes to overexpress themselves will result in a bigger human brain,” Walsh admits. “The first mice in our experiments grew heads so large they could not survive after birth. We’ve done more recent tests in which the overactivity of the gene was toned down, but we have not yet tested the intelligence of these animals.”
Walsh points out that bigger brains aren’t always smarter brains. In fact, bigger human brains can cause mental retardation. “It’s not the size that counts,” he says, “it’s the organization.”
Covering the upper part of our brain is a one-eighth-inch-thick intricately folded layer of nerve cells called the cerebral cortex. This “thinking cap” makes possible unique abilities such as speaking, thinking, learning, remembering, and creating. The cortex of mouse brains stimulated by extra activity of the beta-catenin shows some of this folding, but the organization of brain cells is not as perfect as it is in humans.
“We’re not sure what the punch line is yet,” Walsh comments.
Genes dug out of a hole
When a family with several microcephalic children was referred to him, Walsh became interested in finding out what causes the malady. Working with Ganesh Mochida at Beth Israel Deaconess Medical Center in Boston, they started looking at the genes of people in families with the disease. At the same time, Geoffrey Woods was finding many cases of microcephaly among Pakistani immigrants to England who had been displaced from their homes by construction of a dam. These people included large families that often arranged marriages between close relatives.
Walsh invited Woods to visit Boston during the summer of 2001. While watching a Red Sox game, the two agreed to work together on the problem. From research already done, they knew the approximate location of the gene or genes linked to microcephaly. The next step was to search that location in the human genome, recently mapped by the National Institutes of Health. However, the search found what Walsh calls a “hole” at that place. The genes that his team wanted to find had not yet been mapped.
Walsh and Woods then looked at another version of the genome map prepared by a private company called Celera. Four genes showed up in what was a hole on the government map.
Walsh believed that the ASPM gene would be the one where they’d find the mutation that causes most cases of inherited microcephaly. Woods thought it would be one of the other genes. The two bet a case a beer on the outcome, and Walsh won. “That was last summer,” Walsh said, “and I still haven’t gotten the beer.”
Size and smarts
Mutations of the other genes probably cause different varieties of microcephaly, but for now the spotlight is on ASPM and how it works. The gene produces a protein involved in forming neural stem cells, cells that will develop into brain cells in the cortex. The mutation found by Walsh and Woods apparently disrupts this process, resulting in fewer brain cells and a smaller head.
The beta-catenin protein also works by creating more brain stem cells when its gene is overstimulated.
Walsh points out that ASPM “has the remarkable property that it gets larger as the brain gets larger.” A fruit fly has a smaller protein and fewer brain cells than a mouse, and a mouse boasts fewer than a human. The team plans further investigations that will include the brains of cows, cats, dogs, and chimpanzees to determine how variations in ASPM size might relate to the dramatic changes in cortex size between these different animals.
“The size of the cortex in humans and other species is probably regulated by many different genes,” Walsh notes. “Some of them may be important for the major transition between rodents and primates; others may be involved in changes between chimps and humans.”
Such studies should lead to a more complete understanding of how the brain evolved. In the meantime, what has been learned can be used in genetic counseling. Families who wish to arrange marriages between close relatives can have their genes checked for ASPM mutations. According to Woods, some families in Pakistan now avoid marriages of first cousins. In England, prospective partners often ask to be tested for mutations.
Prenatal diagnosis is also a choice, but terminating a pregnancy goes against the Islamic faith, so it is usually not an option.
“In the future, we also hope to develop drugs that can be custom-tailored for each microcephalic child,” Walsh comments. “Drugs already in use show some promise, such as ritalin, now given for attention-deficit hyperactivity disorder, and tacrine, now taken for Alzheimer’s disease. The more we know about genetic causes of mental retardation, the more drugs of the present – and drugs that come along in the future – can be targeted to specific problems of learning and memory.”
As for manipulating genes to make human brains bigger and smarter, as Walsh says, there’s no punch line yet.




