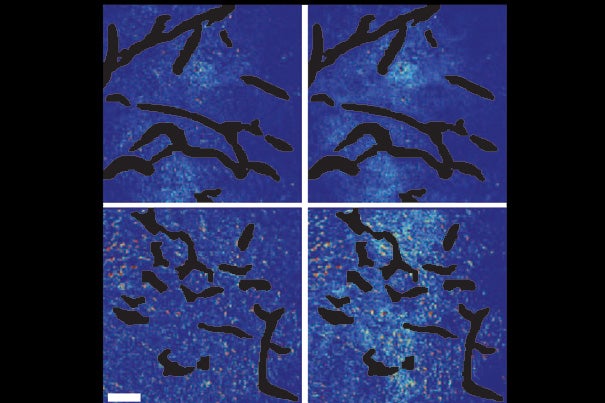
A new study shows that combining angiogenesis inhibitors and nanomedicines only improves cancer treatment when the nanomedicines are at the small end of a size range. Top panels show the control setups. Bottom panels show mammary tumor tissue after normalization of blood vessels. Few of the large nanoparticles are visible in the bottom left panel, while the smaller nanoparticles have penetrated well, as seen in the bottom right panel.
Courtesy of Vikash Chauhan/Nature Nanotechnology
Size matters in drug delivery
Tumor study reveals size limitations for new drugs
Combining two strategies that are designed to improve the results of cancer treatment — angiogenesis inhibitors and nanomedicines — may only be successful if the smallest nanomedicines are used.
A new study led by researchers at the Harvard School of Engineering and Applied Sciences (SEAS) and Massachusetts General Hospital (MGH) has found that normalizing blood vessels within tumors, which improves the delivery of standard chemotherapy drugs, can actually block the delivery of larger nanotherapy molecules.
“We found that vascular normalization only increases the delivery of the smallest nanomedicines to cancer cells,” says lead author Vikash P. Chauhan, a graduate student in bioengineering at SEAS. “We also showed that the smallest nanomedicines are inherently better than larger nanomedicines at penetrating tumors, suggesting that smaller nanomedicines may be ideal for cancer therapy.”
The results have been published in Nature Nanotechnology.
Angiogenesis, the tumor-driven creation of new blood vessels, provides growing cancers with a food source — but it also provides a potential channel for drug delivery.
The problem is that the vessels supplying tumors tend to be disorganized, oversized, and leaky. These abnormalities prevent the delivery of chemotherapy drugs to cells that are not close to the tumor vessels. The leakage of plasma out of blood vessels also increases pressure within the tumor, further reducing the drugs’ ability to penetrate the tissue. Fortunately, drugs that inhibit angiogenesis can reduce some of these problems in a process called vascular normalization.
“Anti-angiogenic agents are prescribed to a large number of cancer patients in combination with conventional therapeutics,” explains principal investigator Rakesh K. Jain, Cook Professor of Radiation Oncology (Tumor Biology) at Harvard Medical School and director of the Steele Laboratory of Tumor Biology at MGH. Jain is also Chauhan’s Ph.D. adviser.
The combination of standard chemotherapy drugs and normalization therapy has previously been shown to improve the effectiveness of treatment on some types of cancer.
New nanomedicines, on the other hand, are designed to exploit the abnormality of tumor vessels. Nanomedicines, despite the name, are actually about 10 to 100 times larger than standard chemotherapy drugs — too large to penetrate the pores of blood vessels in normal tissues, but still small enough to pass through the oversized pores of tumor vessels. Because nanomedicines generally cannot penetrate normal tissues, they are expected to cause fewer side effects.
The question in the Harvard-MGH study was whether vascular normalization would help or hinder the delivery of nanomedicines to tumors. The researchers found, through both theory and in vivo experimentation, that it depends on the size of the nanomedicines.
Their mathematical model predicted that inhibiting angiogenesis would simultaneously reduce the size of the pores in the blood vessels and relieve pressure in the tumor, allowing small particles to penetrate.
Confirming this experimentally in a mouse model of breast cancer, the investigators showed that vascular normalization (using an antibody called DC101) improved the penetration of 12-nanometer particles but not of 60- or 125-nanometer particles.
They treated mice with implanted breast tumors either with DC101 and Doxil, a 100-nanometer version of the chemotherapy drug doxorubicin, or with DC101 and Abraxane, a 10-nanometer version of paclitaxel. Although treatment with both chemotherapeutics delayed tumor growth, vascular normalization with DC101 improved the effectiveness only of Abraxane and had no effect on Doxil treatment.
“A variety of anti-cancer nanomedicines are currently in use or in clinical trials,” says Chauhan, who completed the work at MGH. “Our findings suggest that combining smaller nanomedicines with anti-angiogenic therapies may have a synergistic effect and that smaller nanomedicines should inherently penetrate tumors faster than larger nanomedicines, due to the physical principles that govern drug penetration. While it looks like future development of nanomedicines should focus on making them small — around 12 nanometers in size — we also need to investigate ways to improve delivery of the larger nanomedicines that are currently in use.”
Additional co-authors of the Nature Nanotechnology report are Triantafyllos Stylianopoulos, John Martin, Walid Kamoun, and Dai Fukumura of MGH; and Zoran Popovic, Ou Chen, and Moungi Bawendi of the Massachusetts Institute of Technology (MIT).
The work benefited from a long-term collaboration between Harvard, MGH, and MIT that explores the use of quantum dots as a biocompatible fluorescent marker in medical studies.
Support for the study included grants from the National Institutes of Health and the Department of Defense.
— Adapted from an earlier release by Sue McGreevey, Massachusetts General Hospital.